Scaling Object Lesson #2: Water Electrolyzers For Hydrogen Production
We learned about vertical scaling in the 1st article in this series:
…and about horizontal scaling or “numbering up” in the 2nd:
Now we’ll use these tools to examine the scaling future of an extremely important decarbonization technology: electrolyzers for producing hydrogen from renewable electricity.
My readers will know that I think hydrogen is a massive decarbonization problem, and that I think we must focus our efforts on making green (electrolytic) hydrogen to replace the 98.7% of the stuff that is made from fossils without carbon capture- the ultra-black hydrogen that dominates the market today.
In a decarbonized future, we’ll need a lot of green hydrogen, even if we aren’t stupid enough to waste any as an inefficient vehicle or heating fuel! I estimate that 90 of the current ~ 120 million tonnes/yr of hydrogen we use today, is durable in a decarbonized future. And in reality we’ll need more than that, because there are some uses for hydrogen as a molecule which are also a very good idea: for instance, using H2 to replace the carbon monoxide used in the direct reduction of iron ore to iron metal.
Replacing that 90 million tonnes of H2 per year would take a monumental effort. Optimistically, it would take 4500 TW h of green electricity– more than twice as much as all the wind and solar made on earth in 2019. Given that today we’re making less than 0.03% of world H2 production by the on-purpose electrolysis of water, we’re really at ground zero on the important task of decarbonizing hydrogen itself.
If we had renewable electricity available to us at 100% capacity factor, we’d need only ~ 513 GW worth of electrolyzers to make that hydrogen- but since renewable electricity isn’t available with such high capacity factors, we’ll really need 1000-1500 GW of electrolyzers.
How much on-purpose electrolyzer capacity is there on earth today? Less than 1 GW. We need to increase electrolyzer capacity by at least 3 orders of magnitude and probably more.
Hmm: how can we apply what we learned about vertical and horizontal scaling to this most important decarbonization problem?
Electrolyzer Basics
There are two main water electrolysis technologies which are front-runners today: the alkaline electrolyzer, and the proton exchange membrane (PEM) electrolyzer. They differ in details, features, benefits, disadvantages etc in important ways, but for our purposes we don’t need to worry about that. Let’s look at the basic components of an electrolysis system.
The electrolyzer itself consists of a “stack” of electrolysis cells, each with an anode and a cathode (and many other parts, depending on which technology). Cells are arranged in physical parallel, meaning that each cell is fed water/electrolyte and the products (hydrogen and oxygen gas) are collected from each cell.
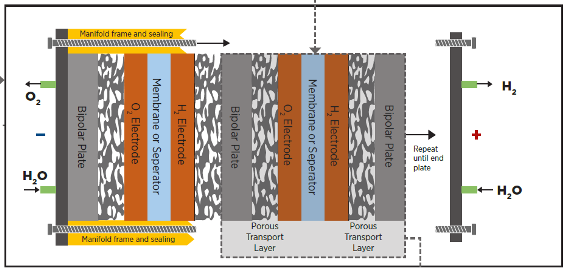
(schematic of an electrolyzer stack- image credit, IRENA)
The cells may be arranged electrically in series, parallel, or series/parallel arrangements to allow us to select DC current and voltage inputs of a manageable level. High currents mean big conductors, expensive power controls, and high ohmic losses- high voltages mean lower currents, but also the potential for currents flowing where we don’t want them to- so we are involved in a balancing act.
Electrolysis is an area-based process. The key parameter for electrolyzer design is current density, i.e. the current, in amperes, which flows through each unit of electrode/cell area. Lower current density means lower voltage per cell and hence higher efficiency, but also means more capital cost per unit of H2 production (or power input) because each unit of electrode area produces less value (less hydrogen) per unit time.
While we could build complete cells individually, and connect each one up to the others with individual tubes and wires or buss bars, that would be very expensive- and we’re smarter than that. Designs vary, but you won’t go far wrong as a basic mental model to think of a stack of plates held together with draw bolts, with internal manifolds and process connections at each end, arranged rather like a plate and frame heat exchanger.
It is advantageous to produce the hydrogen product under pressure, so that less mechanical compression is needed prior to transport or storage of the bulky gas. While pressure forces the equilibrium the wrong way (back from H2 toward water) which costs us some voltage, it also makes the gas bubbles smaller and hence leaves less electrode area blocked by nonconductive gas. Smaller bubbles mean lower current density and hence higher efficiency- to a limit. That means we get a certain amount of gas compression out of an electrolyzer basically “for free” in energy terms- very desirable! Unfortunately, pressure acting on a unit of area generates a force that wants to separate our plates and make the electrolyzer leak- so the bigger we make each plate, the stiffer it must be and the larger and more numerous the bolts that draw the plates together. Ultimately, this basic physics puts practical limits on how big we can make each plate.
Balance of Plant
The electrolyzer stack or stacks are only part of an electrolysis plant. Everything outside the stack which supports it, is called the “balance of plant” or BoP.
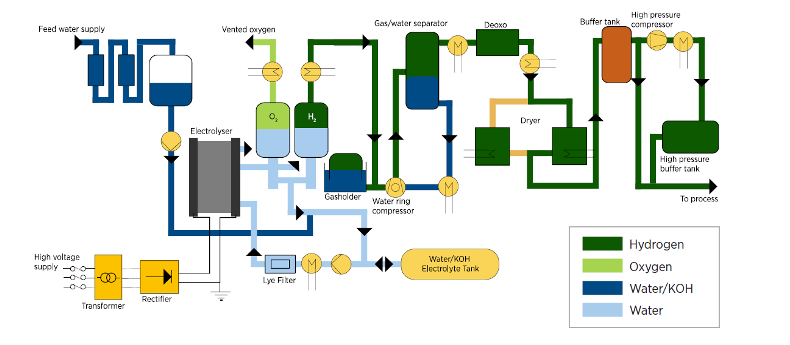
(balance of plant for an alkaline electrolyzer: image credit, IRENA)
Each electrolyzer needs a supply of pure water- at least 9 kg per kg of H2 produced. You can use freshwater or seawater, but in either case you must use reverse osmosis to purify it first- a process which takes a trivial amount of electricity (only 0.035 kWh/kg of H2 even starting with seawater) relative to the 50 kWh/kg it takes to make 1 kg of H2 from water. Impurities in the water can (significantly) sap efficiency by carrying stray currents, can make products like chlorine which contaminate the product gases and destroy materials of construction, deactivate cell catalysts etc. Water purification is a no brainer here- and trying to electrolyze dirty or saline water as a way to make hydrogen, is a fool’s errand.
Electrolyzers need DC electricity of controlled current and voltage. Large variable output DC power supplies referred to as “rectifiers” (but much more complex than a bunch of diodes!) are therefore required, along with all their safety gear, measurement instrumentation and controls, and heat removal systems (because these power supplies are not 100% efficient).
Electrolyzers themselves are not 100% efficient, which means that they convert some of the electricity they are fed, into heat. While a little heat is good (improves efficiency), too much wrecks materials of construction or boils the feed water. Accordingly, heat removal systems are required, and the amount of heat generated at scale is a) is not trivial and b) generally produced in places which already have excess energy available, and which hence have little use for low-grade heat. That means we need pumps and heat exchangers to manage this heat, and yet more heat exchangers to reject this heat to the atmosphere.
The product hydrogen is saturated with water vapour and also contains some oxygen. Generally the hydrogen is passed over a catalyst which burns out the oxygen, forming more water. Drying is accomplished in stages with compression and cooling, and for some processes, more complex drying (based on regenerable adsorbents) may be required. So here too, we’re talking about catalyst beds, heat exchangers, compressors, refrigeration equipment, adsorbent beds etc.
The product oxygen is usually vented, but if it is to be compressed and monnetized, it too needs drying, hydrogen removal and compression.
Finally, the product(s) need to be compressed for transport or storage. Whereas electrolyzers might operate at 30-70 bar, storage is generally at 250 bar or higher. That’s more compressors, piping and storage tanks.
“Outside Battery Limits” (OSBL) Equipment and Infrastructure
An electrolysis plant is like any chemical plant making any other chemical product, in that there are support systems outside the “battery limit” of the chemical plant itself. Some examples include:
- electrical substations, switchgear etc.
- Control and data acquisition system and human/machine interface
- pipeline connections, trailer loading facilities
- water supply and wastewater management
- emergency relief systems, flares, vent stacks etc.
- Buildings or weather enclosures
- facilities for operators or maintenance staff
- roads, parking, civil works
That’s just a partial list- but every such project has to take the OSBL into account, and pay for it.
Scaling of Electrolysis Systems
Assuming that what we want is to make hydrogen as cheaply as possible per kilogram, how should we proceed with scaling up an electrolysis system?
From the discussion about the basic physics of a cell and cell stack, a few things are clear:
- cells are repeating identical units consisting of parts which we might mass produce
- making each cell larger in area will allow each cell to consume more current and hence produce more H2
- making each cell larger in area makes it more likely to leak and requires stronger materials
- arranging cells in fluidic parallel in stacks makes more sense than building innumerable tiny cells and connecting each one
The first point gives us some hope that Wright’s Law can come to the rescue. It is entirely possible to imagine cell components being made in automated factories, and then robotically assembled into cells, and cells into stacks, in a true mass-production environment. And as we do that, with every doubling of production, we should expect the units to get cheaper.
It’s fairly clear that cells of larger electrode area are going to be desirable, but that larger cells will require stronger materials and will be more likely to leak. An optimum size should exist, but that size will vary depending on many things, including the limitations of our mass production scheme.
It’s also fairly clear that it would be desirable to stack up as many such cells into a “stack” as practical, so we have as few stacks as possible- but that there will likely be a practical physical maximum based on mechanical properties, fluid mechanics, and dimensional limitations for transport etc.
And so, the electrolysis industry has concluded. While stack sizes are pushed upward yearly, right now the biggest single stack has a capacity on the order of about 10 MW of input power. A 1 GW electrolyzer therefore would consist of 100 such stacks, arranged in physical parallel, likely in a number of “trains”.
So: what’s our scaling conclusion about electrolyzer stacks? I think we can conclude:
a) Wright’s Law will likely be applicable to the stack
b) since just to replace black hydrogen, we’ll need to increase the capacity of electrolyzers on earth by at least 1000x, there’s lots of room for Wright’s Law to run down the cost of each stack
c) both the cells and the stacks of cells will likely have an optimum size, but people will be motivated to increase that optimum size by clever design
d) the optimum size of stack will require that multiple stacks be “numbered up” to achieve plants of sufficient scale
a) and b) are good, hopeful signs for future electrolyzer stacks to become cheaper with time- assuming somebody will pay for the initial stacks which are expensive per unit of production and not mind doing so.
d) however means that projects of substantial size will involve lots of stacks in physical parallel, and that means that there will be limited economy of vertical scale for the electrolyzer portion of the project
What about the balance of plant?
That consists of “tanks and pumps and sh*t”, as my old boss used to say whenever he was confronted with something that looked complicated and scary, but wasn’t really. Conventional stuff, nothing magical. Nothing we shouldn’t be able to scale vertically to kingdom come. And as we scale that stuff- as an electrolysis project gets bigger in capacity- we should expect the marginal cost of the balance of plant to drop per kg of H2 for the good reasons given in my first article in this series. However, we should also expect nearly zero Wright’s Law benefit in relation to the balance of plant. The same for the OSBL: as a proportion of cost per kg of H2, it should drop as the scale of the project increases, but there should be little to no Wright’s Law learning curve to save our bacon. It’s not like pumps, tanks, heat exchangers, buildings, parking lots, electrical substations etc get cheaper as we make more of them- we’ve made too many of them already, and getting to the next “doubling” takes decades.
The Current State of Electrolyzer Scaling
The major players in electrolyzer manufacture have grown up making fairly small units. Prior to the most recent hydrogen #hopium pandemic, the whole role for electrolysis was either to make high purity hydrogen for specialist applications, or to make quantities of hydrogen to rescue users of small quantities of the gas from the high prices being charged by gas suppliers for tube trailer deliveries from a commercial hydrogen plant. Anybody who needed hydrogen at a meaningful scale, simply bought their own small SMR and made it themselves instead from much cheaper fossil gas.
Often, electrolysis supported pilot projects or provided hydrogen as a utility to an existing facility. Accordingly, many of the electrolysis suppliers designed modular products, often based around “seacans” (shipping containers) which served as both environmental enclosure and support for the equipment. Some units were self contained in a single container, while others required several containers for a complete unit.

(container modular electrolyzer system: image credit- Plug Power)
What do you think about the idea of mass producing containerized complete small electrolyer systems in containers? What are the economic prospects for such a design?
In my opinion, based on the type of analysis we’ve used in the past two articles, the prospects for such an approach are very poor indeed. I see this design as a hangover from the industry’s history.
While the stacks will still have Wright’s Law cost learning benefit, complete containerized packaged systems could benefit from factory fabrication but likely would have minimal to no Wright’s Law learning based cost reduction benefit. The economy of vertical scale for the balance of plant would be abandoned due to the very small maximum scale of complete units stuffed into containers of a limited size, and the resulting marginal capital cost per kg of H2 would be higher than necessary. Furthermore, shipping containers, though themselves mass produced (for use AS shipping containers…) are an inefficient use of steel, an inefficient way to enclose space relative to constructing a building, they offer poor access for operation and maintenance, and are (greatly!) suboptimal in size relative to (considerably) larger modular frameworks which can ALSO be moved by road and sea to most locations. In modular systems, the optimal size of a module is the biggest piece you can move down the road without excessive “heroics”, so you have to re-assemble as few modules as practical on site: this maximizes one of the key benefits of modularization, which is minimizing expensive site work by doing as much in the factory as practical.
The ridiculous extension of this approach is the proposed path to scale of Enapter, a company developing a technology called anion exchange membrane electrolysis. Though the AEM technology has an interesting combination of desirable properties of both alkaline and PEM units, with some of the downsides of each removed, Enapter’s publicly announced strategy is to mass produce complete 2.3 kWh electrolyzers, each including its own complete balance of plant. Thousands of such factory mass-produced units would be physically paralleled to produce an electrolysis project at scale. Sadly, I think this is an object lesson in how not to scale up an otherwise potentially promising technology. It shows to me a lack of understanding of fundamentals of engineering economics.
Fortunately, there are smarter people in the electrolyzer space, both among the market leaders and a number of their rivals. They have a clear view of what it would take to make electrolysis cheap enough to make cheap green hydrogen at scale, and are pursuing development projects for equipment that are consistent with good engineering economics. Some of them are even smart enough to be Spitfire Research customers! But in the interest of maintaining confidentiality, I won’t mention their names, unless they want to “out” themselves in the comments.
Insights for the Future Cost of Green Hydrogen
To become truly inexpensive, truly green hydrogen requires the following things:
- a) very cheap renewable electricity available at high capacity factor, which means hybrids of wind and solar in places which have sunny days and windy nights
- b) projects of very large vertical scale
- c) electrolysis plants that are inexpensive at scale
- d) to make green hydrogen production into a business, you also need a way to get the green hydrogen to market.
(Sharp readers will notice that I never mentioned efficiency- and that might make some people suspect I’ve been smoking some #hopium myself. Why is efficiency not on that list? Because the existing state of the art of electrolysis is already 83% HHV efficient, which sadly is only 70% LHV efficient- though hard to afford at that low current density. The incremental benefit from 83% to 100% on an HHV basis- the limit for water electrolysis set by thermodynamics- is, in my view, much less important than the other factors!)
To achieve a), you need large hybrids of wind and solar, located far away from electricity markets (or else you’ll simply make and sell electricity instead!)- locations such as western Australia, Chile etc. That unfortunately collides with d)- locations far away from electricity markets are also far away from places which can use hydrogen, and moving hydrogen across transoceanic distances is a bear of a problem. Accordingly, those projects will need to make something you can move, such as ammonia, direct reduced iron,, methanol or the like. And, hopefully, we’ll be smart enough not to waste those materials as ways to make hydrogen again…
To achieve c), you need somebody willing to buy expensive electrolyzers- and the expensive hydrogen they produce- sufficiently to get electrolyzer production far enough along the Wright’s Law learning curve to make the cells and stacks cheap enough. That’s possible but it will take deep pockets- our public pockets. So it’s my hope that we aren’t stupid enough to waste any of that precious, expensive but truly green hydrogen on dumb uses that are better served by electrification directly or via batteries, or which can be eliminated, or which can be solved more effectively by other means (biofuels etc.)
What you also need is b), ie for the balance of plant and OSBL to be done at large vertical scale, because it won’t be subject to Wright’s Law and hence won’t get cheaper as a result of learnings. And sadly, there’s only so much that vertical scale can do to reduce the costs of this part of the cost of a total hydrogen plant.
Will green H2 get cheaper? Absolutely! But only because it is insanely expensive at the moment.
Will it get cheap enough? Depends on what you mean by cheap enough, and for what purpose.
Will it get cheap enough to replace black hydrogen? That depends more on what we do in relation to carbon taxes and emission bans than it does in relation to the scaling of electrolysis technology. I dearly hope so- our lives are depending on it, if we want to keep eating that is. Dumping CO2 to the atmosphere for free, or nearly free, is a giant subsidy on the backs of our childrens’ futures that we must END, TODAY.
Will it get cheap enough to go head to head with the electricity it is made from? No. That should be obvious.
Will it get cheap enough to use as a fuel, for transport or heating? I doubt it. I think there are better options that will achieve decarbonization of these functions, at far lower cost to society. That goes even moreso for so-called e-fuels derived from hydrogen. Fighting thermodynamics all the way back from water, CO2 and electricity is a fool’s errand- one that should be undertaken only if we are both rich and desperate.