A Coke can that has been dissolved in sodium hydroxide, revealing its internal plastic liner (photo credit- YouTube)
TL&DR: destroying reduced metals/metalloids like aluminum and silicon is a really, really dumb way to make hydrogen. It is energetic vandalism, and generates unnecessary toxic emissions to generate a low value product.
A shorter, more perfunctory version of this argument is available in an article for TreeHugger by Lloyd Alter, which quotes a LinkedIn post I made about this three years ago that somehow vanished from LinkedIn without explanation.
https://www.treehugger.com/why-make-hydrogen-aluminum-6752088
Lots of idiots are talking about making hydrogen by reacting metals like aluminum or metalloids like silicon, with water. Why? Because hydrogen, despite being a very low cost commodity chemical, 99% of which is produced from fossils like natural gas and coal without carbon capture, is a vector for #hopium. Its largely pretended and heavily exaggerated future as an energy vector and fossil fuel substitute, gives it a certain cachet with people who don’t understand chemistry. Foolishness and wasted money- largely public money- is the inevitable result.
In order to explain the stupidity of these notions, we have to look carefully at what chemical reduction is, and why it’s so important.
A key thing to remember in chemistry is “LEO says GER”: loss of electrons is oxidation, and gaining electrons is reduction. Oxidants (examples being oxygen, chlorine, hydrogen peroxide etc.) are things that accept electrons readily, and reductants (examples being carbon, hydrogen and metals like sodium and magnesium) are things that give up electrons readily. Electric currents can also be used to force things to oxidize and other things to be reduced- the perfect example is the electrolysis of water, where hydrogen ions gain electrons (are reduced) to form hydrogen gas, and oxygen ions are oxidized (lose electrons) to form oxygen gas.
Reductants Are Important
Thanks to the autotrophs about 2.1 to 2.4 billion years ago, we’re swimming in an atmosphere which is about 20.9% oxygen. Since we live in a swamp of a fairly strong oxidant, we don’t tend to find reductants just lying about. Because- what’s another name for a reductant?
A fuel.
A fuel is, in the typical way we think of the word, something you can react with oxygen to make energy (though we also talk about uranium and thorium as nuclear “fuels”). Fire is, frankly, optional.
And yes, that makes metals “fuels”, too- though some of them burn in air only with difficulty.
Winning Metals From Their Ores
Very few metals are found in the reduced state in nature. Only copper, silver, gold and some of the platinum group metals are found uncombined with other elements on earth, and generally in quite tiny quantities too. All the others are found in an oxidized state, as metal ions in compounds with other elements, particularly oxygen and sulphur. And reducing those metals to their native metallic state, takes considerable energy. That energy obviously has to come from somewhere.
The method of reduction used in producing a metal varies depending on its ore, what other metals and non-valuable rock (gangue) it is found with, and what process is used to purify the metal prior to reduction.
Carbothermic Reduction
Iron is an example of a metal produced by carbothermic reduction. Iron ores consist of iron oxides containing iron in the Fe+2 and Fe+3 oxidized states. Iron has historically been reduced by reacting these oxides with carbon monoxide produced by the partial oxidation of carbon. Originally it was charcoal which we used in our pottery fires, and later it was metallurgical coal. The reaction between CO and iron oxides is exothermic and produces CO2. About 4% of iron today is reduced by reacting it with mixtures of carbon monoxide and hydrogen made from natural gas- a process referred to as the direct reduction of iron (DRI).
The reductant (the thing that is oxidized) is carbon, and the products of that oxidation are heat and carbon dioxide. The oxygen in this case is partially supplied as combustion air, and partially comes from the oxygen bonded to the iron in the ore.
Silicon is made in a similar method: carbon and silica (SiO2, quartz sand) are reacted with one another in a submerged arc furnace, yielding molten metallurgical silicon and carbon dioxide. Boron, another metalloid, can be produced in a similar fashion.
Obviously in a decarbonized future, other methods will be required. In the case of iron, hydrogen can be used to replace carbon with electric resistance heating required because the reaction between iron oxides and hydrogen is endothermic, not exothermic. Sadly, those processes don’t work with silicon or boron, so our choice there is either carbon capture and storage, or the development of other methods. But while we could emit fossil CO2 to the atmosphere without a care in the world, it was all good fun.
“Smelting”
Some metals, including copper, zinc, lead, nickel, cobalt and iron, are also found in the form of sulphides. Sulphur, like carbon, can be oxidized with oxygen, producing sulphur dioxide. Sulphide ores of these metals can be heated to high temperatures under oxidizing conditions, in some cases yielding molten metal rather than the metal oxide, and SO2. The SO2 can be further reacted with oxygen to produce SO3, which reacts with water to make sulphuric acid H2SO4. Of course in the old days, we vented the SO2 and it produced sulphuric acid in nearby lakes, rivers and soils, dropping the pH, mobilizing phytotoxic metals and rendering the surrounding countryside into a hellscape. That’s been frowned upon since the 1980s, but in the old days, piling ore on top of a bonfire consisting of hundreds of thousands of trees was considered a valid method of metal production.
Hydrometallurgy and Electrowinning
When nickel, copper, cobalt etc. are found as oxides rather than sulphides, “smelting” won’t do the trick because there’s no reductant available. In some cases, carbothermic reduction is possible, but in others, it isn’t. Accordingly other methods of reduction are used. The ore concentrates are often dissolved in acid to produce a solution of metal ions. The metal ions are then separated from one another by various means, and then each metal is reduced. While hydrogen can be used as a reductant in some cases, electrowinning is the typical method used today. Electrowinning involves “plating” the metal from aqueous solution electrolytically, onto a starter sheet made of pure metal. Electrowinning is extremely energy intensive,but serves to both reduce the metal and to considerably purify it by a combination of preferential electrolysis and preferential crystallization. As an example, copper is electrowon from a sulphuric acid solution:
At the cathode: Cu+2 + 2e- à Cu (metal)
At the anode: 2H2O + 4e- + 4H+ + O2(g)
The result is the production of very pure copper plus the regeneration of the H2SO4 used to produce the copper sulphate solution.
Reactive Metals
Aluminum, magnesium, lithium, sodium, potassium, calcium, titanium, zirconium and other reactive metals are generally uncooperative. Some of them are very easily oxidized, and to reduce them we need more violent methods. While magnesium can be produced from its carbonate by a carbo-ferro-thermic reduction under vacuum (called the Pidgeon process), most reactive metals can’t be produced either by carbothermic or aqueous electrolysis means.
These reactive metals are all produced in one of two ways:
- Electrolysis of a molten salt of the metal, or
- Reduction using a more strongly reducing metal (i.e. magnesium is frequently used for this purpose). Titanium and zirconium, for instance, are made by reducing their chlorides with magnesium metal to form magnesium chloride, which can be recycled to magnesium and chlorine by molten salt electrolysis. As my old friend Alex Grant is fond of saying, “titanium is just two magnesiums in a trench coat”- titanium is produced by “magnesio-thermic reduction”
Aluminum is the key example here, as it is produced in giant quantity and is the 2nd most important structural metal next to iron.
Aluminum is produced from ores containing aluminum oxide (alumina) such as bauxite. The alumina is extracted and purified by the Bayer process (currently largely fossil fueled today like most industrial processes requiring heat), and then mixed with a fluoride containing flux to reduce its melting point. The flux-alumina mixture is melted at around 950 C and electrolyzed.
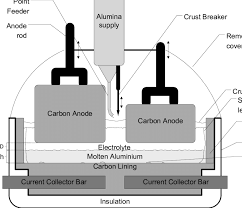
The electrolyzer, a large refractory-lined box, has a cathode layer on the bottom and inserted from the top. The anodes used are generally carbon, made from a mixture of petroleum coke and “pitch” (residuum), and the cathodes are graphite. The anode, where oxygen is generated, serves two purposes: it carries away electrons liberated by oxygen generation to the electrical circuit, and it reacts with that oxygen to produce CO and CO2. The resulting carbothermic reduction carried out by the anodes reduces the electrical energy required to carry out the reduction, at the cost of both CO2 emissions and the emission of fluorocarbon compounds of increasing toxicological concern.
While inert anodes have been developed (Google the company Elysis for more details), the resulting reductions in CO2 emissions come at a cost of a considerable increase in the electrical energy intensity of aluminum production.
Aluminum production is already extremely electrical energy intensive. Figures vary, numerous LCA studies can be reviewed for details, but to a first approximation, aluminum production requires about 8.5 kWh/kg (largely heat) for raw materials production (including about 4.5 kWh/kg aluminum for the consumed carbon anodes’ embodied energy) and about 15.5 kWh/kg (largely electricity) for electrolysis. At 24 kWh/kg in total, which is only 86 MJ per kg, the figure is considerably lower than figures published for aluminum’s embodied energy, which can range from 190 to 230 MJ per kg (53 to 64 kWh/kg) of primary energy- though it appears that this is largely a result of the fact that much aluminum production in the world is not made using hydroelectricity or nuclear power, but rather using fossil fuels (whose primary energy is only partially converted to electricity).
Regardless how you slice it, aluminum is an extremely energy intensive metal. The carbon intensity varies, but even if absolutely GHG free power is used for electrolysis and also for the Bayer process (which currently is nearly 100% fossil fueled), 0.45 kg of carbon anodes are combusted per kg of aluminum produced- that alone represents 1.65 kg of CO2 per kg of aluminum. Typical figures for aluminum production today are on the order of 15 kg CO2e/kg aluminum.
Taking a soda (pop) can with a mass of about 14 grams and a volume of 330 mL, the energy intensity is particularly stark. 24 kWh/kg (i.e. the low heat + electricity figure, ignoring the upstream conversion of heat to electricity) means the embodied energy of the can is about 0.34 kWh (mostly electricity) and its embodied emissions of its raw material alone are about 0.21 kg of CO2e. If you were burn 330 mL of gasoline in a 25% gasoline generator, you’d have about 0.33 L x 8.9 kWh/L LHV x 25% = 0.73 kWh. So the next time you’re tempted to throw an aluminum can into the garbage rather than the recycling bin, consider that you’re throwing away embodied energy amounting to the work potential of about ½ that same can full of gasoline. You either need to stop using aluminum cans, or make sure that they’re properly recycled- even if that means carrying it around with you for a while until you find a recycling bin.
The heat of formation of alumina from aluminum and oxygen is -1676 kJ/mol. That’s the ultimate embodied energy of aluminum from a chemical potential energy standpoint. That’s 1.67 MJ /3.6 MJ/kWh/0.027 kg/mol = 17 kWh/kg. You might be tempted then to divide 17 kWh/kg by 24 kWh/kg from the energy of production figure and come up with an efficiency of 71%- but that would involve comparing a product (17 kWh of mostly heat) against a feedstock of considerably higher value (24 kWh of mostly electricity). While the current (faradaic) efficiency of modern aluminum electrolysis cells is about 90-95%, their energy efficiency is closer to 45%. These devices spend most of their energy grinding thermodynamic work (electricity) up and spitting out not aluminum metal, but heat.
(The data used here is a little dated, but the paper itself is absolutely rich with detail and very easy to read- much less of a mental bludgeon than a typical academic LCA study)
https://www.aceee.org/files/proceedings/2003/data/papers/SS03_Panel1_Paper02.pdf
Fortunately, aluminum is also extremely easy to recycle and has a very high value in the recycling stream. Recycling aluminum waste reduces the energy intensity of its use by about 95%.
So- What’s Wrong with Using Metals to Make Hydrogen?
Well, the same thing that’s wrong with burning metals as fuels. And yes, people are proposing to do that, too.
The foregoing should demonstrate fairly clearly that we expend a lot of effort and energy, and generate lots of emissions (toxic and GHGs alike), in the effort to win metals from their ores.
If we were to burn finely divided aluminum, we’d make alumina dust and heat. However, aluminum rapidly self-passivates by producing a protective layer of alumina which reduces the reactivity of the underlying metal. The same happens when we immerse aluminum in water.
However, if we use a method to disrupt the alumina film (gallium or indium or mercury will do this), we can cause aluminum to react with water to produce aluminum hydroxide or oxy-hydroxide, and hydrogen. We can also react aluminum metal with sodium hydroxide solution to produce sodium aluminate (NaAlO2) and hydrogen.
Well heck- you could use sodium instead! Chuck sodium metal into water and you get sodium hydroxide and hydrogen- no fancy gallium required!
Why would we want to do this? After all, aluminum hydroxide or oxy-hydroxide, and sodium aluminate, are what we started from in the Bayer process when we were purifying bauxite to make alumina! Same deal with sodium- it’s made by electrolysis of sodium chloride, forming sodium metal and chlorine gas- not by electrolysis of molten sodium hydroxide which is also possible- but still, you can likely see where I’m going here.
The desire to do this crazy thing comes from a) the problems of hydrogen
storage and distribution due to its low energy density per unit volume and b) hydrogen’s incredibly simpleminded seductiveness as a potential low carbon fuel.
https://www.linkedin.com/pulse/green-hydrogen-fuel-zombie-meme-paul-martin-gpplc
Why carry around low density gaseous or slightly higher density ultracryogenic liquid hydrogen, when you can carry around aluminum, a catalytic amount of (extremely rare and expensive) gallium, and can find water lying about in ponds all over the place? You can make hydrogen on demand!
Well…you mean aside from the extreme emissions intensity, and the thermodynamic stupidity of the whole thing?
The excuse is “we can use waste aluminum”, and “alumina is valuable”.
What is Waste Aluminum?
If it’s aluminum metal scrap, in metallic sections of decent dimension, that can be easily recycled- right back into aluminum products. The recycling rate of aluminum metal is already very high. If it’s pure aluminum (which we rarely use), it can be recycled into aluminum metal and used again straight away. If it’s an alloy, it can be used to make aluminum alloys by judicious blending with pure aluminum, which is how we recycle every other metal.
This is no different than how we recycle all other metals: copper, for instance. Copper wire scrap isn’t used to make copper wire, as the purity requirements of conduction applications are too high to satisfy with scrap. So instead, we use wire scrap to make pipe and tubing. And we use pipe and tubing scrap, which might be contaminated with lead or tin or antimony from solder, to make brasses and bronzes. What do we do with scrap brasses and bronzes? What we can’t use to make more brasses and bronzes, we might bite the bullet and send back to a copper refinery so the copper value can be recovered again by electrowinning. What we don’t do is chuck the whole works in acid and electro-win it every time, because that would be energetic and emissions insanity!
The real issue is that we use aluminum sometimes in product forms that are difficult to recycle. Some examples are aluminum foil and thin aluminum packaging articles like disposable pie plates. These items tend to burn (oxidize) rather than melting, unless they are carefully separated and compressed first.
We also use aluminum in micro-thin layers- vapour-deposited aluminum on potato chip bag film, for instance, which can be only a few nanometres thick but which still provides incredible value as an oxygen and light barrier.
Those uses, plus aluminum that is “lost” because people chuck their cans and other products into a landfill stream that isn’t properly sorted. While these losses are real, and have emissions associated with them that we should be concerned about, most of the uses are either a) very small users of aluminum in mass terms or b) are only available in theory, rather than in practice, as they are not segregated from the waste stream or attached to much larger masses of other material (i.e. plastic).
Are there heaps of aluminum alloy scrap laying around, going unrecycled, that could become a ready, cheap source for making hydrogen?
No, there aren’t. Point me to some, and I’ll point you to a business opportunity!
And remember: if an aluminum waste stream is too contaminated with other metals to be used in making fresh aluminum alloy products, then any oxide or oxy-hydroxide made from that material is going to be useless too.
The Thermodynamics of Making Hydrogen From Aluminum
Ultimately we’re talking about a process for water electrolysis to produce hydrogen, except we’re doing it indirectly using aluminum. Instead of producing oxygen from water, we’re producing aluminum oxide, hydroxide or oxy-hydroxide- but to close the loop, we’ll have to produce aluminum again from these materials. It’s not more complex than that, irrespective of what project proponents might tell you!
Water electrolysis is already possible at efficiencies as high as 95% on the HHV basis (which is 80% on the LHV basis- the 6.1 kWh/kg of heat of condensation of the product water is lost in any device that attempts to produce work (electricity) from hydrogen). Real electrolyzers you can afford to buy are closer to 50-55 kWh/kg H2 all in, or about 60-66% LHV efficient. So- why on earth would we bother with aluminum as a middleman? If melt electrolysis alone runs around 45-50% electrical energy efficiency, why would we want to substitute THAT for water electrolysis? And remember, we still have to collect up all this wet oxy-hydroxide from points of hydrogen use and bring it back to a Bayer plant to calcine it- assuming it’s not too contaminated for re-use.
What’s Really Going On Here
This is really just another hydrogen export scam. People confronted with the fundamental, immutable properties of the hydrogen molecule, which make it a poor choice of fuel even if it were possible to make it efficiently for that purpose, end up inevitably reaching for a way to convert hydrogen into something else which is easier to transport. And by so doing, they always end up in another, deeper thermodynamic hole. A hole which they fall into, losing energy on the way in, and have to climb back out of – using energy to do that, too.
It’s true with all methods proposed for hydrogen storage or remote hydrogen generation. Doesn’t matter if you reach for ammonia, methanol, liquid organic hydrogen carriers, metal hydrides either as reagents with water to make hydrogen or as chemi-sorbents for gaseous hydrogen, or chucking aluminum or sodium or sodium borohydride into water. They all make the thermodynamic cycle path longer by introducing “middlemen”, which add cost and complexity and which waste energy.
The Bottom Line
Anybody proposing to use aluminum “waste” to make hydrogen is an energetic vandal. The same is true of anyone planning to use reduced silicon waste, but even worse because silicon production is purely carbothermic at present. The resulting energy cycle efficiency is terrible, making water electrolysis look wonderful in comparison, and it’s craptastic compared with batteries. The toxic emissions footprint of the resulting hydrogen will also be disgusting.
And so what will you see them do? They’ll try to justify it either via the (non-existent) market value of the alumina/oxy-hydroxide product, or they’ll compare it against a strawman- using the hydrogen as a transport fuel for instance, as a recent MIT study tried to do. When you compare against the real reference technology- using battery EVs for transport instead for instance- the result is still clear as a bell. Hydrogen is a dumbass fuel for transport, regardless how you make it.
If you do happen to have tons of reduced metals laying around that you want to throw away just for their energy value, you could easily enough make a primary (non-rechargeable) battery out of them. At least then you’ll get thermodynamic work (electricity) rather than energetically useless hydrogen.
Can You Make Hydrogen From Other Wastes?
Another popular myth is that you can take a fossil fuel, such as waste plastic, and convert it into a non-fossil fuel- whether that be hydrogen, a liquid fuel produced by pyrolysis, or a liquid fuel produced from H2/CO syngas. Sadly, that’s a greenwash. It’s incineration, once removed. The details of that particular greenwash are handled in another of my articles:
https://www.linkedin.com/pulse/waste-energy-fuels-great-greenwashing-machine-paul-martin
While you could make hydrogen from waste biomass, in fact the syngas from that waste biomass is worth more than the hydrogen you’d get by water-gas shifting the CO to CO2. We’ll need lots of biomass derived fuels for shipping and aviation in a decarbonized future, and it makes way more sense to start with CO than with CO2.
Can You Make Hydrogen as a Byproduct?
A large fraction of hydrogen is currently made from petroleum. Why? How? It’s a byproduct of cracking and dehydrogenation reactions, where alkanes are made into olefins and aromatics. Some of that hydrogen is so mixed with other gases that it is simply sent to the fuel gas stream and burned, but that’s changing.
Another major source of hydrogen today is as a byproduct of chlor-alkali manufacture. When we electrolyze saltwater to make sodium hydroxide and chlorine, bleach etc., more hydrogen is produced today than all the “on-purpose” electrolysis of water (so-called green hydrogen) on earth. Some of that hydrogen is wasted by venting it, some is burned as a fuel, and some is used to make hydrogen chloride. Some is used to offset fossil hydrogen generation.
The pyrolysis of methane has also become a popular thing in the public imagination, since my former client Monolith commercialized the process of making carbon black and hydrogen from methane.
Methane has a value that is only equal to its heat energy content, because at commercial scale, pretty much the only other thing that you can make from it that has any value is syngas. In a decarbonized future, fossil methane will be almost worthless. That has led some people to conclude that it would make sense to make hydrogen from methane with carbon capture and storage (so-called “blue” hydrogen, which I call “blackish blue, bruise-coloured hydrogen”)
https://www.linkedin.com/pulse/blackish-blue-bruise-coloured-hydrogen-spitfire-research-inc
Others realize that CCS is expensive, and that throwing away solid things is cheaper than burying giant quantities of gases. Accordingly, they’ve concluded that it might make sense to pyrolyze methane just to make hydrogen, throwing away ½ of the feed energy and 3/4 of its mass into low value applications. My bet is that they’re wrong.
https://www.linkedin.com/pulse/hydrogen-methane-pyrolysis-paul-martin-vacnc
Certainly companies like Monolith, who make a truly valuable carbon product, and Transform who make a valuable chemical (acetylene) instead of carbon, have an easier shot at having a real business than those who want to sell only hydrogen. But if you must sell carbon as a valuable product to have viable economics, methane pyrolysis will simply never be a major source of hydrogen. As my article linked above makes clear, all you need to understand that is a very basic mass balance.
Finally, it’s possible to make hydrogen as a byproduct of making other chemicals, including sulphuric acid. If you do it the right way, the result is a dramatic (~50%) reduction in the electrical energy required to make a kg of hydrogen from water. My client Peregrine Hydrogen is doing just that. In their case, the same customers want the hydrogen and the sulphuric acid, so it has obvious market traction.
Disclaimer: this article has been written by a human, who found the section about aluminium production to be particularly hard to write- there’s too much contradictory and poorly referenced data available to a cursory search, and an in-depth review of numerous LCAs was beyond my needs in this paper. If you find good references which identify mistakes in my analysis, I’ll be happy to go back and correct my work, within reason, especially if it meaningfully affects the conclusions.
If, however, I’ve taken a dump on your pet commercial idea, feel free to contact my employer, Spitfire Research Inc., which will be quite happy to tell you to piss off and write your own article.