Hydrogen to Replace Natural Gas- By the Numbers
Update: my peer reviewed article in Energy Science and Engineering summarizes many of the issues in this particular blog post:
https://scijournals.onlinelibrary.wiley.com/doi/10.1002/ese3.1861
UPDATED: April 6, 2023
There’s been a lot of talk recently about hydrogen as a replacement for natural gas. The scheme is to gradually add H2 to the natural gas grid, with the H2 being made from water using “excess” renewable electricity when it’s available. But ultimately, there are people who think we should have pure hydrogen supplied to our homes instead of natural gas, using the same piping and distribution network that we have now. In their minds, all we’d have to do is to re-jet all our boilers, furnaces, stove cooktops and ovens and we’ll be away to the races. No need to abandon all that expensive capital- we’ll just change the fuel! We’ll be burning colourless, odourless hydrogen, making only water vapour, and global warming will be one step closer to being solved.
Sounds great! Where do I sign?
Hold on- not so fast!
In case you prefer video to reading (I read far faster than I can watch anything, but to each their own!) Rosemary Barnes did an excellent video interview with me that used excellent graphics to get my points across- and asked excellent probative questions too. Well worth a watch- and the detail is here in the article you’ve already clicked on if you want to understand the issue more completely.
Replacing Gas With Hydrogen is An Inefficient Use of Energy
The first and most obvious criticism of this scheme is efficiency. It doesn’t matter if you start with natural gas or electricity, the best you can do is to convert about 70% of the feed energy (lower heating value (LHV) of methane, or kWh of electricity) into LHV of product hydrogen. Best case. If the alternative is to use natural gas or electricity directly, hydrogen brings nothing but loss to that equation.
Obviously the whole idea here is to eliminate the fossil greenhouse gas (GHG) emissions associated with the burning that’s happening at your end of their pipe. Hydrogen offers the option to do that. You can start with bio-methane from anaerobic digestion, so the CO2 you emit when you make hydrogen is just part of the natural carbon cycle. Or you can capture all or part of the CO2 produced when making hydrogen from fossil natural gas at the hydrogen plant, or by pyrolyzing the methane and selling carbon as a byproduct for uses other than burning, or you can avoid the CO2 entirely by making the electricity you feed your electrolyzer from wind or solar, nuclear, hydro, geothermal etc. These are all ways by which you could end up with a fossil GHG emission-free fuel for your burner- ideally that is, assuming you could afford it.
You could of course feed the grid with methane from biogas instead- but while I’m convinced biogas will be an important fuel for those fuel uses we really do need in a post-fossil future, nobody should try to convince you that there will be enough biogas EVER to just replace existing natural gas supplies- or even a small fraction of those supplies. So if you want to keep your burners, and not emit fossil GHGs, hydrogen seems like your only option. And that’s exactly what the natural gas industry is telling governments all over the world.
Of course, these gas companies and electrolyzer suppliers are not giving their advice without self-interest in mind. They are starting from the position that they need to stay in business, and you need to keep your burners- fair enough! The obvious alternative is to replace your burners directly with electricity and cut out the lossy hydrogen middleman, but that would leave them out of business. For home heating, and even for domestic hot water, a heat pump will not only save you the 30% conversion loss to hydrogen, it will also give you about 3 kWh worth of heat for every kWh worth of electricity you feed. Far, far more efficient. But not cheap- the heat pump is going to cost you quite a few dollars- and while renewable electricity is getting cheaper by the day, grid electricity still sells at a large multiple of the cost of natural gas per unit of energy- because carbon taxes are inadequate, and because in some places, fossil fuels still power the grid.
For your cooktop, an induction heater will give you even better performance than a flame- you may have to throw out a few of your old aluminum pots and pans, but otherwise you’ll likely be very happy with that change. And your oven will do nicely with a plain old resistance heater- with much better temperature control.
Remind me what we need a fuel gas for again, exactly? I know only one answer to that- right now, natural gas is a very, very cheap fuel IF you ignore the fossil GHG emissions from both its production and distribution and its burning. Displacing natural gas use from home heating is going to be a tough struggle regardless how we do it- because the alternatives are going to cost more, at least initially.
https://www.linkedin.com/pulse/home-heating-electrification-paul-martin/
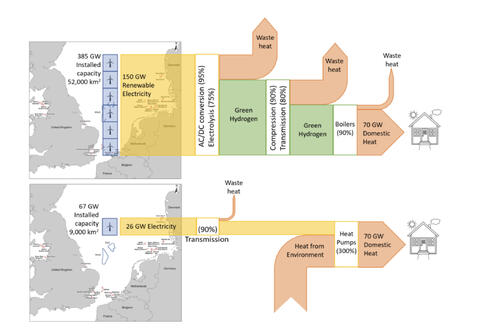
Note that the figure above is almost accurate- the distribution losses for hydrogen are exaggerated a bit due to a misinterpretation of a piece of data in a peer-reviewed article by Ulf Bossell, but even so- the efficiency of heatpumps may not be 5-6x better than hydrogen, but it’s at least 4-5x better…
Hydrogen, on the other hand, isn’t a cheap fuel, period. And it should be obvious that it can NEVER be as cheap as either the natural gas or the electricity from which it is made.
Hydrogen Distribution is Lossy and Expensive
Even assuming that you were so nostalgically attached to your gas appliances that you couldn’t part with them, the gas industry would still need to overcome some serious problems that aren’t being discussed, before hydrogen starts flowing through the natural gas grid.
If we’re going to make hydrogen, whether it’s “blue” hydrogen made from natural gas with carbon capture and storage, or “green” hydrogen made from water using renewable electricity, it still has to get from where it’s made to your house. And it’s not as simple as just changing what flows through the pipes.
Compression- the Deal Killer
To move any gas economically, it needs to be compressed. And it turns out this is the big problem with hydrogen distribution- it’s the reason that 85% of hydrogen produced in Europe, for instance, travels basically no distance to where it’s consumed, because it’s made right on the same site or right next door.
Natural gas is about 8.5 times as dense as hydrogen, and dense gases are easier (more energy efficient) to move than less dense ones. Hydrogen partially makes up for that fact by being more energy dense per unit mass- about 3 times as much as natural gas. But, sadly, the work (mechanical energy) needed to drive a compressor is related linearly to the number of moles of gas we compress, rather than to their mass or volume per se. It also depends, more weakly and in a more complex way, on the ratio of specific heats of the gas- which, as it turns out, makes a minor difference (in favour of natural gas) which increases with increasing compression ratio. But when we compare the LHV of hydrogen per mole to the LHV of natural gas per mole, we find that natural gas is about 2.9 times as energy dense in molar units. Another way to put it is that it takes about three times as much energy to compress a MJ’s worth of heat energy if you supply it as hydrogen than if you supply it as natural gas. And this, folks, at least in part, explains why we don’t move hydrogen around much by pipeline. Instead, we move natural gas to where hydrogen is needed, and build a hydrogen plant there. (see the end of the article for the proof)
That 3x increase in the work of compression not only costs energy, it would also cost a gas utility big money, since it would mean that every compressor in their network would need to be replaced with a new unit with 3x as much power, and also physically larger- with 3x the suction displacement. And since hydrogen is so notoriously leaky, the hydrogen volumetric flowrate is higher for a given heat flow in the pipe line etc., the compressors would need to be totally different machines- considerably more expensive ones.
Hydrogen is, already, round numbers, about 37% best case in cycle efficiency when starting and ending with electricity. Whereas natural gas and electricity are roughly the same cost and efficiency to distribute on a per unit energy basis, hydrogen is going to cost about 3x what natural gas costs in lost energy, just to move the gas. And since the downstream equipment is only 50-60% efficient at producing electricity again, you’re going to have to move roughly twice as MUCH hydrogen energy to destination to do the same job as if you moved electricity instead. That’s forgetting about the extra capital cost that would also need to be spent.
Pressure Drop in Piping- A Wash
You’d think that you’d suffer an additional penalty moving hydrogen through piping once you’d gotten it up to the desired pressure- that was certainly my first impression. But as it turns out, the answer to that question is quite complex, and it depends on what conditions you run the calculations at. Hydrogen is less dense, less viscous, and more energy dense per unit mass than natural gas. But when you run the pressure drop calculations at the sorts of velocities and pressure drops used in pipelines which carry gases long distances (where pressure drops are on the order of 5 psi per mile of pipe, rather than the 5 psi per 100 ft of pipe that might be typical in a chemical plant’s piping), hydrogen and natural gas come out nearly even at a given rate of LHV heat delivered per hour down a pipe of given size. That does change at different points in the distribution system, and to a 1st approximation, the average works out to an existing gas pipe being able to carry about 90% of the energy n the form of hydrogen that it could carry if it were fed the average natural gas it was designed for. The velocity will be about three times higher, but the density is 1/8.5x as much, and together with the modestly lower viscosity, the factors nearly cancel one another out. However, since every kWh of energy lost due to friction in the pipeline has to come from a compressor, that still means that hydrogen costs about 3x as much per unit of energy to move from source to destination in a pipeline.
“Line Pack”- What’s That? Another Problem…
As I promise my readers, I EDIT my articles when they teach me new things or point out my mistakes. And a knowledgeable connection brought to my attention this rather major problem that is a result of hydrogen’s lower energy density per unit volume. “Line pack” is the name given to the amount of natural gas stored in the piping distribution system itself. And unless we increase the pressure of the distribution system- which we cannot do without new pipe- we will lose that storage. A typical gas system apparently can handle about 3-4 hours of average demand just using stored gas in the lines. Pure hydrogen, being 1/3 as dense in energy per unit volume, would reduce that to ~ 1 hour. That could mean a giant difference in distribution system reliability, the frequency and duration of outages, and the ability of the grid as it exists to handle variations in demand- the big spike when everybody gets home, cranks up their furnaces or boilers and turns on their cooktops for instance.
I’m already aware that sometimes, subdivisions out-grow the rate at which the gas utilities can install new lines to them. Accordingly, some utilities evaporate liquid natural gas from tanks into points downstream of the “bottleneck” in order to keep the furnaces and cooktops humming through peak hours. Doing that with hydrogen would be very expensive and very dangerous, given that liquid hydrogen takes about 40% of the energy IN the hydrogen just to liquefy it, boils at 24 Kelvin (24 degrees above absolute zero- liquid methane boils at a balmy 112 Kelvin or -161 C)- and as a liquid it is still only 71 kg/m3- methane is about 420 kg/m3 in comparison as a liquid.
Piping and Equipment
If you don’t heat it up too much, hydrogen is quite safe to carry in mild steel piping- even up to fairly significant pressures. The much talked about “hydrogen embrittlement” isn’t a factor in relation to hydrogen gas handling for soft mild steel or low alloy steel piping such as what is used in most chemical plant piping.
However, natural gas pipelines- particularly the pipelines carrying natural gas long distances or underwater- are not made from mild steels. They’re made from harder, strong steels- and those steels are, according to many reports, susceptible to hydrogen embrittlement or other hydrogen related damage mechanisms, particularly in their welds and heat affected zones- even at fairly modest pressures and temperatures.
According to credible reports written by natural gas distribution utilities themselves, such as this excellent one:
-most of the high and medium pressure natural gas distribution system would need to be totally replaced to handle pure hydrogen. (see p.12 of that reference, where it says this in as many words- and these guys, who own the pipes, should know best!) That’s a massive cost- especially to spend on a change to a fuel which might be better replaced with electricity anyway.
Note that hydrogen damage and hydrogen embrittlement are complex metallurgical topics, and that nascent hydrogen (hydrogen atoms generated by electrochemical action such as during corrosion) causes damage that molecular hydrogen cannot until a combination of high pressure and high temperature make that possible. But the reports about H2 compatibility problems with pipelines used for natural gas is quite well demonstrated, by people who know this issue far better than I do.
Here’s another reference, from AIGA standard 087/20:
From Standard AIGA-087/20 (Asian Industrial Gases Association) Section 4.2.1- Metals
“… For high pressure applications, carbon steel shall be used with caution. Carbon steels with high-carbon content and high-strength, low-alloy carbon steels are susceptible to embrittlement and crack propagation. The use of carbon or alloy steels requires control of tensile strength, heat treatment, microstructure, and surface finish as well as initial and periodic examination for inclusions and crack-like defects when in cyclic service”
And another, from Sandia National Laboratories:
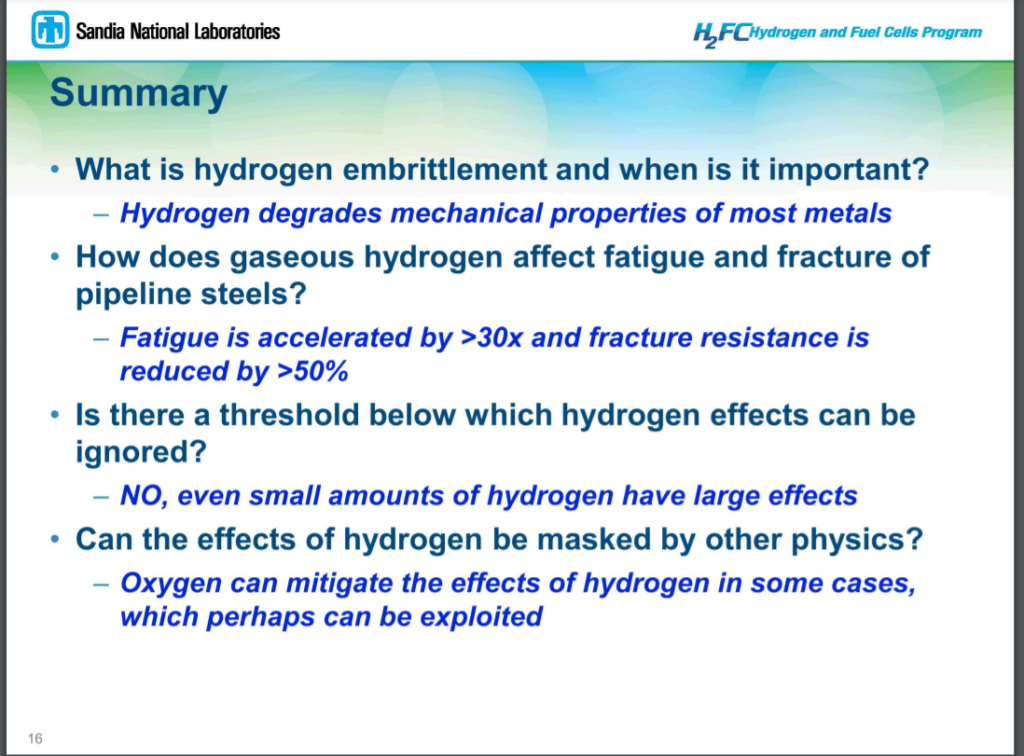
A webinar I attended (September 21, 2022) on the platform “Mission-Hydrogen”, confirmed these concerns. (A video recording of the webinar and of the presenter’s slides is available). The lecturer was Dr. Milos Djukic of the University of Belgrade, a senior fellow of the European Structural Integrity Society, whose summary paper on the issues related to hydrogen damage and embrittlement mechanisms in steels and alloys has been cited over 100 times. In the talk, Dr. Djukic confirmed that 1-5% H2 is enough to cause worries about hydrogen assisted fatigue crack growth, that fatigue can be accelerated by more than 10 times (indeed Dr. Djukic confirms the rate may increase up to 30 times), and fracture resistance can be reduced by more than 50%, and that all the normal high yield stress API grades of pipeline steel are susceptible. Dr. Djukic concluded in his talk as follows (emphasis is his):
“It is widely believed that existing gas pipelines that are retrofitted for transportation of natural gas-H2, CH4-H2 and N2-H2 gas mixtures, and repurposed for 100% H2 transport, are viable options and safe for long term future usage. However, despite the fact that gaseous H2 transport via existing gas pipelines is a low-cost option for delivering large volumes of H2, there is a serious threat of hydrogen damage and catastrophic failure, particularly for old-aged gas pipelines, after future long-term H service. “
UPDATE April 2023: a recent study paid for by German gas and water association DVGW proves, by careful measurement, that the materials used in gas pipelines- even low yield strength versions- suffer from accelerated fatigue cracking and reduced fracture toughness, even in some cases at fairly low hydrogen partial pressures. However the study claims that it’s OK, because the cracking doesn’t happen faster and the fatigue resistance doesn’t drop sufficiently to fall outside the limits of dedicated hydrogen piping design code ASME B31.12- a code that fossil gas pipelines are NOT designed and fabricated to.
Under the hydrogen design code, the design pressure of an existing fossil gas line would need to be de-rated to perhaps 1/2 to as little as 1/3 of its original rating if it were switched to carry hydrogen. That’s going to have a very large impact on the energy carrying capacity of those lines, likely necessitating either twinning or replacement of any transmission line switched to hydrogen. And it stands to reason that “twinning” would need to be in the cards anyway, as in any realistic transition scenario there will be a time when a transmission system would need to supply both hydrogen AND fossil gas to its customers. Hmmm…the notion that you can re-use this infrastructure meaningfully is looking less and less promising the more we look into it!
https://www.linkedin.com/pulse/german-gas-pipelines-fundamentally-suitable-carrying-hydrogen-martin/
The low pressure distribution system is mostly made up of low carbon steel and HDPE pipe, and you can run hydrogen through that easily enough.
(UPDATE: even HDPE may have some problems with hydrogen, per NREL’s recent report (10/2022): see https://www.nrel.gov/docs/fy23osti/81704.pdf)
However, piping designed not to leak natural gas can leak a lot of hydrogen due to hydrogen’s low density and high diffusivity. Intact HDPE piping and the seals and other “soft goods” used in the distribution system are quite permeable to hydrogen. And, sadly, stenching agents such as the thiols (mercaptans) used in natural gas to help detect leaks, may be used in hydrogen for burning, but not with hydrogen to be used to feed PEM fuelcells such as those used in vehicles. The catalysts in those fuelcells are extremely sensitive to sulphur compounds like that. Given hydrogen’s extremely wide explosive range- any mixture between 4% and 75% hydrogen in air is explosive- and its low ignition energy- the lack of a stenching agent to help you detect leaks seems a very challenging problem for distribution of this fuel to homes and businesses.
Hydrogen/Natural Gas Mixtures
The initial projects all try to smooth over these problems by mixing a little H2 into natural gas instead of making the big leap to pure hydrogen. And when you hear about “replacing 20% of natural gas with hydrogen”, you’d think that would make a big difference!
Think again.
A 20% mixture of H2 in natural gas is a 20% mixture by volume. That mixture has only 86% of the energy of an average natural gas, meaning that you’d have to burn 14% more volume of gas to make the same number of joules or BTU of heat. The savings in GHG emissions are nowhere nearly 20%- they’re closer to 7% just looking at the burning (assuming perfectly carbon free green hydrogen), and less than that when you consider the compression and pressure loss noted above. Such a reduction would already cause heat content sensitive users to scream, so forget about going to 30% H2! For a given amount of energy delivered, a 20% mixture of hydrogen in natural gas would take 13% more energy to compress and would lose about 10% more pressure per unit length of pipe than if you were to stick with natural gas- because the gas has to flow faster, and yet isn’t sufficiently lower in density to compensate. Those factors would eat some of your GHG emission savings. And while industrial users would be protected- they pay per BTU or joule of LHV or HHV they are delivered by the gas company- some users could be shortchanged since they pay per unit volume instead.
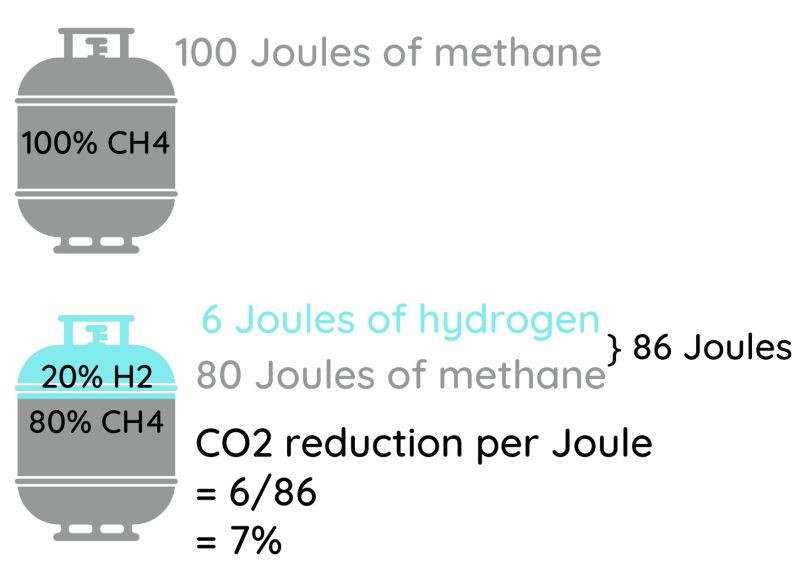
(Image Credit: Rosemary Barnes, from her Engineering With Rosie video, link previously provided)
Of course, to get ANY meaningful reduction in GHG emissions, you need to use “green” hydrogen (made by electrolysis using fossil-free energy). That doesn’t exist meaningfully in the market at present- it is too expensive. So what is brought to the rescue? So-called “blue” hydrogen, made the normal way, from fossils, but with carbon capture and storage (CCS). Sadly, that approach makes only muddy blackish-blue, bruise-coloured hydrogen at best, as this paper by Howarth and Jacobson published in Environmental Science and Engineering makes clear. Fossil advocates have pooh-poohed the paper because it uses methane emissions higher than they’d like to admit to, and because it uses the 20 yr time horizon greenhouse potential of methane relative to CO2 which is 86x CO2. But even if you use the sensitivity analysis in the paper to trim back the estimated methane leakage, and you imagine that all “blue” hydrogen production will use new oxy-blown autothermal reformers so carbon capture can be more complete, “blue” hydrogen appears to be a very poor strategy for decarbonization. It is, in contrast, an excellent strategy in the view of the fossil fuel industry, because their objective is to stay in business, not to decarbonize anything really.
But What About “Hard to Decarbonize Industries”?
Another excuse we hear for the need for hydrogen to replace natural gas is for “high temperature industrial heating”. For some reason, people just seem to assume that because we run some equipment right now by burning fuels, we cannot instead use electricity. The examples of steel and cement-making are frequently brought up, but there are many others.
Here I have to bring in what I do for a living. I design and build pilot plants, which are prototype units to test new chemical processes. These plants can vary from tiny lab units to quite large facilities that would look to the average person like any other real chemical plant. But the one thing that a pilot plant will almost entirely without exception be missing is any fired equipment. There are exceptions, but aside from the function of disposing of waste streams of combustible materials, every function that is accomplished on a commercial chemical plant using fired equipment, is done using electricity instead on a pilot plant. Why is that?
Many reasons:
1) Electricity is far safer and easier to control than fire, particularly at the small scale. Electric heating provides rapid, accurate control and reduces hot spots, reduces risks to materials of construction etc.
2) Electricity costs more than fuel as a heat source, but the energy cost of a pilot plant is seldom the most important factor to its operators.
3) Fired heaters generally need air emissions permits and may require stack gas testing- costs which the pilot plant avoids by using electric heating.
4) To heat a stream to high temperatures using a burner, you are left with a high temperature flue gas exiting the unit. Chemical plants make use of that hot flue gas to heat up numerous other streams to keep it from going to waste- or use it to make steam to drive equipment or keep things hot. On a pilot plant, it is just not worth the trouble of doing that kind of heat integration
5) Fired equipment is more expensive than electrically heated equipment
6) When you need the highest temperatures, sometimes electric heating is the only feasible option.
In steelmaking, the real need for hydrogen isn’t for heating at all- electric arc furnaces for steelmaking are already quite popular. Hydrogen is needed to replace the chemical reductant carbon monoxide made from coal coke, which is used to reduce iron oxide to iron metal. There are direct electrochemical reduction methods also under development, so it’s possible we could also make steel without using hydrogen at all.
In many other applications, electric heating could easily be used to eliminate the need to burn fuels. It would however require modification to major pieces of equipment, which might have a considerable cost. But if the alternative is to spend a multiple of that cost on hydrogen made FROM electricity, that savings can pay for quite a bit of capital.
In fact, if approached with a fresh sheet of paper and without a firebox on your head, most applications in industrial heating currently served with fire for cost reasons (because fuels are cheaper, as long as you can dump fossil CO2 to the atmosphere), could easily be converted to electric heating instead.
All we really need is to price fossil carbon emissions at a rate high enough- and durably enough- to make the associated capital investments worthwhile in economic terms for the affected industries.
Hydrogen Toxic Emissions
You will frequently hear the old trope that when you burn hydrogen, you get nothing but water! While that IS true if you “burn” hydrogen catalytically in a low temperature fuelcell, it is NOT true in general terms.
Burning ANYTHING in air results in nitrogen oxides (NOx) being generated by reaction between oxygen and nitrogen in the air. The higher the combustion temperature, the more NOx you generate. And the more H2 you add to a natural gas mixture, the higher the resulting free air combustion temperature will be- and hence, the higher the NOx emissions will be.
NOx consists of two important nitrogen oxides and one transient species. NO2 is the dangerous brownish gas which is toxic, produces “acid rain”, and is a photochemical smog precursor. It is however quite water soluble (hence the acid rain) and so it isn’t environmentally persistent.
N2O (nitrous oxide) isn’t toxic- it’s used as an anaesthetic and it may even be produced in our own bodies. It is however a powerful, persistent GHG with a 100 yr greenhouse potential of 300x that of CO2.
Industrial combustion equipment burning hydrogen or H2-rich mixtures can be fitted with selective catalytic reduction (SCR) units, which react NOx with hydrogen to produce N2 and water again. Not so with home appliances though- it is fundamentally impossible with things like cooktops for obvious reasons, and it is economically impractical with devices like furnaces, rooftop heating units, hot water heaters and heating boilers too.
This issue seems to be conveniently forgotten. Natural gas burning in homes is already a major source of indoor air pollution and apparently also a major cause of juvenile asthma. Hydrogen will make that worse, not better, relative to natural gas.
Flame Visibility
Don’t let the stupid “hydrogen olympics” fool you. Hydrogen flames are rich in the UV and emit very limited amount of visible light. They are visible only at night, unless the H2 is contaminated with something. The Olympic flame was contaminated deliberately with sodium carbonate, giving it the eerie orange glow from the spectral emission lines of sodium.

Hydrogen for Seasonal Energy Storage
Another argument that I frequently hear is that because of the double whammy of greater energy need for heating and lower solar power production in winter, we’ll need hydrogen to make up the shortfall. We’ll need to make vast quantities of hydrogen in summer, and store it in salt caverns until winter. While stored fuels of some kind are likely a useful part of an emergency response plan in any post-fossil fuelled future, it is to me a non sequitur that just because it’s possible to use hydrogen for this purpose, that doing so would actually make energetic or economic sense. Methane, whether from biogas or even fossil natural gas, seems a more logical choice as a gas to store, given that we already have strategic and emergency stores of natural gas in place. And we could just as easily store up a year’s worth of biogas methane as we could find a way to make hydrogen in excess in summer.
Green hydrogen’s chief economic problem as an energy storage medium is the cost of electrolyzers and storage equipment- and as we’ve seen in this paper, distribution cost isn’t going to be as low as some expect either. Multiplying the low capacity factor of a wind or solar production unit by another seasonal capacity factor of say 0.5 or less, doesn’t add up to a low capital cost per kg of hydrogen stored. This stored fuel would be very expensive indeed, even if the power itself were quite cheap.
Why Are We Doing This Again?
NREL’s document issued in October 2022 about hydrogen blending (see https://www.nrel.gov/docs/fy23osti/81704.pdf) contains a very good summary of the challenges involved in replacing fossil gas with hydrogen, including this excellent table:
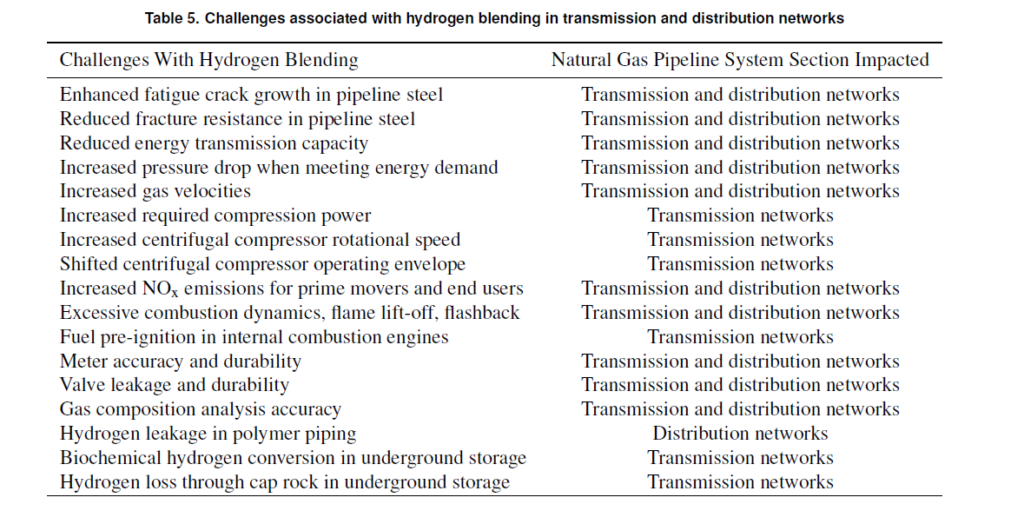
In summary, it seems to me quite clear that hydrogen’s role as a replacement for natural gas has more to do with a need for gas production and distribution companies to stay in business by having something to sell, than any real GHG emissions benefit or significant technical need. And if they want to make the necessary investments entirely on their own nickel, to provide truly green or even “blue” hydrogen via an upgraded network to replace natural gas, perhaps that’s OK with me. Sadly, it seems quite clear that their caps are in hand, reaching out to the public sector to fund the necessary infrastructure investments. Personally, my thinking is that this would be throwing good money after bad.
DISCLAIMER: these are my personal opinions, informed by my knowledge and practice of chemical engineering over the past 30 yrs. My opinions are my own, and are not to be confused with those of former employers or current or former clients. I am motivated only by a sincere desire to get us off fossil fuels and by so doing, eliminate fossil GHG and toxic emissions associated with burning them, for as low a cost and impact on society as we can manage. Every article I write is likely to make one or another of my customers angry- you can rest assured of that!
I have made my best effort to be accurate in what I’ve said, doing my own confirmatory calculations. I can provide background on those to anyone who asks. But I’m human, and hence prone to error. I also don’t for a moment claim to know everything there is to know about this subject matter, which is where some people have spent their entire careers. If you can show me where I’ve gone wrong in my analysis or calculations, with references or dependable examples, I’ll gratefully edit my piece to reflect these new learnings on my part.
APPENDIX:
Here’s the abbreviated logic behind why it takes 3x as much compressor energy to move a given amount of H2 LHV as to move the same number of J or BTU of natural gas LHV.
Where a and b are constants, different for each gas, but only a little different between H2 and natural gas, and r is the compression ratio i.e. P2/P1, P1 is the initial absolute pressure and V1 is the initial volume, the work of adiabatic compression is given by a formula of the following form:
W = a P1V1 (1-r(1/r)^b)
Per the ideal gas law, P1V1 = nRT1, where n is the number of moles of gas, R is the ideal gas constant, and T1 is the initial temperature.
Taking gases 1 and 2 of nearly equal values of a and b (to avoid getting results which vary with r), and taking them at the same initial pressure, volume and temperature, it can be shown that:
W1/W2 = ~ n1/n2
Hydrogen has a molar LHV of 240 kJ/mol, and a middle of the road natural gas might have a LHV of 695 kJ/mol. The work ratio is therefore ~2.9:1 for hydrogen versus natural gas, if we were to move a constant number of kJ of LHV per compression stroke, or per unit time.
The actual values of a and b (related to the Cp/Cv ratio) for H2 and natural gas at commercially significant compression ratios adjust this 2.9:1 ratio to about 3:1.