TL&DR: grinding up electricity to make heating fuel is just a way to waste energy and capital, by destroying exergy (the potential to do thermodynamic work). It’s obviously worse than just making hydrogen. It is wasteful, and wasteful means expensive. It also means higher emissions than if you did something sensible, like feeding a heatpump.
There’s been a lot of recent news about projects by a firm called TES, in both the US and Canada. A proposed TES project in former Canadian Prime Minister Jean Cretien’s region of Shawinigan, Quebec, has been making major news here in recent days. No surprise, one of the project’s proponents is Jean Cretien’s daughter, France Cretien Desmarais, is a major proponent of the project.
When I wrote my lengthy tome about the various ideas of using hydrogen as an energy export commodity-
Hydrogen to Replace Natural Gas- By the Numbers
– I looked at all the various options for transporting hydrogen- as a gas, as an ultra-cryogenic liquid, as ammonia, methanol, solid metal hydrides, and in the form of hydrogenated molecules (liquid organic hydrogen carriers). All of these looked pretty stupid, due to capital costs and losses. But one real stinker missed the list- the idea of reacting CO2 with hydrogen to make methane. I had to add that to the article later, because it seems to be mentioned more and more often as people contend with the very real problems of distributing hydrogen itself. The simpleminded notion that you can just change which gas is sold in the natural gas network was put to sleep in another of my articles-
– and that notion too remains alive, like a zombie, kept that way by the natural gas industry who knows that they are out of business post decarbonization without it.
Some clever folks just say, “Why don’t we put biogas into the natural gas network”? And why not? It’s a mixture of methane and CO2, about equal amounts. While most end-use devices won’t like all that extra CO2, removing it should be easy enough- certainly dead easy compared to removing CO2 from the atmosphere at ~400 ppm…
https://www.linkedin.com/pulse/why-direct-air-capture-sucks-good-way-paul-martin/
Sadly, aside from a few places where agriculture is very intense, there simply won’t be enough. And despite the fact that biogas is made from a waste, by the time you have the CO2 out of it, biogas methane costs a multiple of what we are used to paying for fossil natural gas. It’s also distributed across the landscape, so collecting the source material, producing the gas, and inserting it into the existing gas network is no easy task. Small scale makes it expensive, and physical distribution across the landscape means that you either have to transport waste to larger facilities, or build lots of small, capital-inefficient ones.
So of course the bright sparks who think we’ll be positively drowning in green hydrogen because that’s just frigging inevitable, because electrical grids are hard and whatnot (insert other nonsense as you see fit- the green hydrogen crowd has lots of it, and #hopium smokers the world around seem to be gobbling it up)…well, they think maybe we should react that green hydrogen with CO2 and make…methane.
You know, the exact reverse of the process by which substantially all the world’s hydrogen is actually made today. Whereas we run steam or coal reformers to react these fossils with steam over some catalysts to produce, ultimately, CO2 and hydrogen, these folks want to do the opposite- react CO2 with hydrogen to make…methane.
The Sabatier Reaction
We’ve known about the Sabatier reaction, also known in the industry as “methanation”, for a long time. It is commercially used sometimes as a way to back-treat dangerous CO and CO2, which can destroy fuelcell catalysts, to inert methane, as a treatment method in hydrogen purification.
CO2 + 4 H2 ===> CH4 + 2 H2O
Basically, the reaction involves “un-burning” CO2, by reacting off both oxygen atoms with hydrogen and then replacing the vacant bonding sites with even more hydrogen.
The reaction is spontaneous and exothermic- thermodynamics doesn’t mind if we’re dumb enough to want to do this. And if you were to convert 100% of the energy in the feed hydrogen to the higher heating value of product methane, the ultimate thermodynamic efficiency would be about 78%. That’s actually pretty good, considering.
No real reaction goes that perfectly, though. For instance, another reaction we’ve known about for a long time is this one: one of the methanol synthesis reactions:
CO2 + 3 H2 ===> CH3OH + H2O
That one is also spontaneous and exothermic. And its ultimate thermodynamic limit efficiency is 85% on a higher heating value (HHV) basis-not surprising perhaps, because it wastes one fewer H2 molecule than the Sabatier reaction does, making worthless water. But in reality, you can’t do it for any more than about 60% efficiency in a real plant- which is, unshockingly, the approximate efficiency by which natural gas LHV can be converted to methanol LHV in a world scale black methanol plant.
Another way to put that, is that real methanol systems fed CO2 and H2, only get to about 70% of the ultimate efficiency that thermodynamics sets as a limit. Why is that? Well, lots of reasons, but the big one is that conversion per pass sucks, because the real reaction going on is via carbon monoxide and the water-gas shift reaction pathway rather than directly from CO2 to methanol. That means we end up spending a lot of energy making CO and water out of CO2 and H2, and then running the whole shebang around in a loop, cooling it down, removing the methanol and water products and then re-compressing and re-heating the gas to feed it to the reactor again. Real processes don’t approach thermodynamic perfection very often, and the closer you push them toward that ideal, the more capital cost you end up spending trying to recover energy that would otherwise be wasted.
So: why is e-methane seductive: because a) a giant transmission and delivery system exists for the product, which unlike hydrogen, can actually be fed to that network in any proportion desired b) the reaction is fairly brainless, just requiring a series of fixed bed catalytic reactors with heat and water removal between them. Methanol is a lot more complex in terms of the technology and the plant required.
It’s simple, but that doesn’t stop e-methane from being dumbass. We’ll get there.
CO2 Sources Matter!
If we are planning to make methanol (which might be sensible) or methane (which probably won’t be!) using energy entirely in the form of green hydrogen, and we’re planning to use these molecules as fuels, then it matters a lot where we get the CO2 we use for that purpose.
Some focus on the notion that only the source of enthalpy (chemical potential energy) matters. They think it’s OK to capture fossil CO2 produced by burning or, say, black hydrogen production, and get away with the fact that when the fuel is burned, the captured fossil CO2 ends up in the atmosphere anyway.
I don’t.
We need to stop burning fossils as fuels, because we need to stop emitting fossil CO2 into the atmosphere.
Period.
Getting a 2nd pass on CO2 from burning fossils by capturing (some of it) and using (some of) it in a fuels production process is, at very best, a 50% reduction in the carbon intensity of the original fossil fuel use. That’s just not good enough.
So if you want to call your product an e-fuel, you need, at minimum, to be using pure biogenic or (well…no, this won’t happen) direct air captured CO2.
Evaluation of the TES Shawinigan Project
TES has been granted a 150 MW Hydro Quebec block of power- very green, cold water hydroelectric power with very high capacity factor. Power that should be fed to decarbonization efforts in Quebec- real ones, like electrification of transport, or, at least, fed to hungry electricity consumers in the US so they can shut off their coal and gas fired power plants.
TES apparently plan to add a large amount- hundreds of megawatts more in the form of wind and solar projects that they would build themselves.
Their plan is to convert that electricity to hydrogen, and then react it with biogenic CO2, presumably captured from combustion of wood and wood byproducts in the forestry industry, or perhaps from the CO2 portion of biogas methane etc.
Let’s take this sucker apart and see if it makes sense!
Energy Efficiency Chain for e-Methane
First, we start with electricity. Let’s feed it to an electrolyzer which takes 52.5 kWh/kg of hydrogen. That’s 75% efficiency on the higher heating value (HHV basis), or about 63% efficient on the lower heating value (LHV) basis. That’s a little lower than the 70% LHV basis I usually use in these calcs, but I’ve chosen this for two reasons:
1) To be consistent with what David Cebon used in his hydrogen for heating graphic produced for the Hydrogen Science Coalition (below) and,
2) Because in numerous projects done as a consultant in the past few years, I’ve never, ever seen a real electrolyzer quotation which achieved better than this on a total balance of plant basis. Most fall between 55 and 65 kWh/kg.
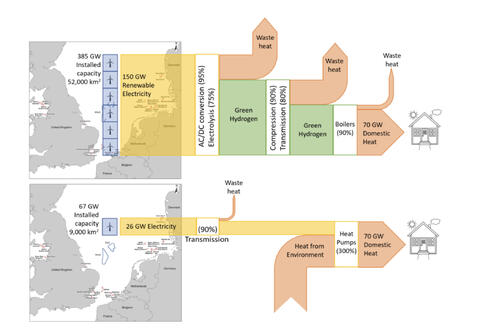
Let’s throw in a 1% grid electricity loss, just to show that we didn’t use a big loss here. This is basically an “islanded” project, consuming its own power and electricity made at a nearyby hydro dam.
We’ll also throw in a 95% efficiency (i.e. 5% loss) for hydrogen compression, storage and losses associated with moving hydrogen around on the site.
Next, let’s apply a 92% efficiency, i.e. an 8% loss, to supply the energy necessary to capture and compress the required CO2. That’s a very, very optimistic figure, based on the 2.1 MJ/kg CO2 which is used to capture 78% of the 3.8 bar partial pressure CO2 at Shell Quest. We’re using that figure despite its obvious optimism, just because the figure is publicly available. Any post combustion CO2 capture, and any capture that requires transport of the CO2, is going to take a lot more energy than that!
Now we feed this hydrogen and CO2 to the Sabatier reactor. And remember, best case that converts 78% of the HHV of hydrogen fed, to HHV of methane. Let’s assume that it operates better than methanol, and achieves 75% of maximum theoretical energy efficiency, with the balance going to yield losses, energy to run pumps and compressors, gas separation equipment, cooling to remove the produced water etc. This is based on optimistic comparison to other processes. Based on a ratio of what their project could achieve at best case using the 78% perfect conversion, and how much methane they think they’ll be making, TES seems to be taking this figure as 85%- but hey, they’re bound to be optimistic about their efficiency, I guess. 78% x 75% = 59% overall conversion from hydrogen HHV to methane HHV.
Now we put the gas into the gas transmission system. Compression for transport is about 97.5% efficient, and then transport/distribution itself is about 97.5% efficient per the ANL GREET model. That’s another 95% efficiency or 5% loss in terms of the gas energy we send to customers.
And now, we feed it to a gas boiler or furnace at 90% AFUE.
If we feed 1 MWh of electricity to the front end, we end up with 0.32 MWh of low grade comfort heat, in a home heated with the resulting e-gas. 32% cycle efficiency- but worse than even that…because we fed work (electricity) and got out heat…
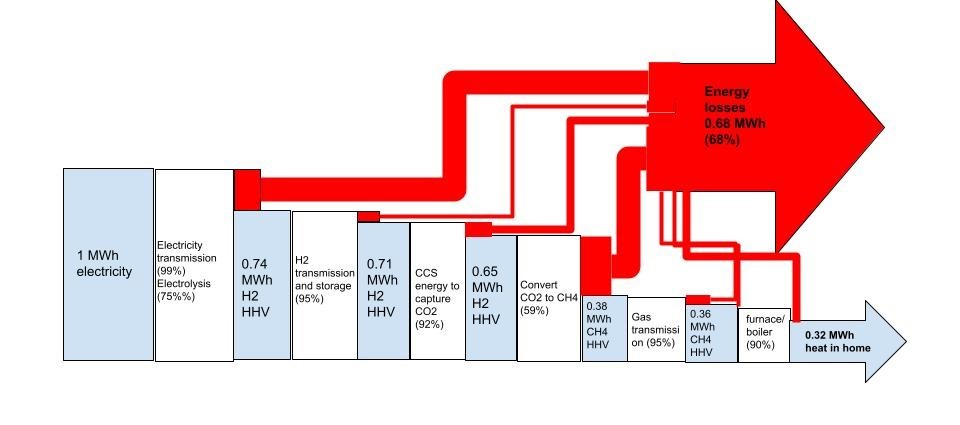
But it’s even worse than that when we consider EXERGY, i.e. the potential to do work. And the best way to consider that, is to look at what else we might do instead.
Let’s say we fed the same 1 MWh of electricity into the electrical grid. Distribution is about 95% efficient based on US average data from EIA (i.e. 5% grid loss)- in fact in Ontario where I live, it’s less than 3% loss. The electricity is now already at your home’s meter, where we feed it to an air source heat pump with a coefficient of performance of 2.5 (we’re not expecting 3 out of a unit in most of Quebec, though most Quebecois live along the St. Lawrence, not way up north- just as most other Canadians live within 100 miles of the US border.
1 MWh of electricity then delivers 2.4 MWh of heat into the home. This one is so simple we don’t even need a Sankey diagram to see how awesome it is.
2.4 MWh divided by 0.32 MWh is a factor of 7.5 times. We can heat 7.5 times as many homes for the same amount of electricity if we just leave the molecular middlemen out of it!
And we haven’t even begun to talk about cost!
What Does This e-Methane Cost?
Well, it’s 7.5 times what it would cost to run a heatpump using the same energy, right? Well, it’s not quite that simple- but almost. In fact, it’s far, far worse than that!
Figures in this section and elsewhere are in Canadian dollars unless otherwise noted. $1 CDN = $0.74 USD as of today.
Figuring this out in detail is a little trickier than doing an efficiency chain diagram. But let’s do it, just because we’re bored!
First, let’s assume that TES gets electricity, on average between the 150 MW they get from Hydro Quebec and the wind and solar they make for themselves, at $0.05/kWh. That’d be pretty amazing, but let’s be hopeful here! That’s $13.89 per GJ of electricity, i.e. considerably more expensive than the ~ $7/GJ Energir is reportedly paying today for wholesale natural gas HHV energy. As of today, the US Henry Hub wholesale price for gas is $US2.78/MMBTU which is about $3.70 CDN/GJ. So already, even at an amazing 5 cents per kWh, that’s a lot of money going down the drain, to waste it as heat!
Now, let’s assume a few figures to estimate the cost of green hydrogen. $0.05/kWh x 52.5 kWh/kg is $2.63/kg H2 just for electricity. Add in a $2 million CDN/MW electrolyzer which lasts 10 yrs, which costs us nothing for operating labour or other O&M, and which is given to us by benevolent people who don’t want a return on their investment- they’re satisfied with simple payback when the electrolyzer turns into a pumpkin. And let’s assume a capacity factor for our power of 100%. It won’t really be that high, because only 150 MW is firmed hydropower. But again, let’s be blissfully hopeful here. That drives up our best case price for green hydrogen to $3.82/kg. That’s $27/GJ for hydrogen HHV.
Doubleplusnotcheap!
Now we feed it to a conversion process which makes it into e-methane at 59% efficiency. We don’t know what Sabatier reaction systems cost, so let’s assume they’re free, and have no other operating costs. The result is still $27/GJ divided by 59%, or about $46/GJ of methane HHV. We also need to supply about 0.32 GJ worth of energy to satisfy other energy losses in the chain, but let’s assume they’re supplied as cheaper 5 cent per kWh electricity. That adds another $4.50/GJ, taking us to, round numbers, a blue sky ridiculously hopeful cost of e-methane to TES- NOT to the consumer- of $50/GJ. 50/7 is about 7.1 times the cost of gas currently being paid for by Energir, wholesale.
Relative to the heatpump…well, it’s a lot more than 7.5 times more expensive for energy. Remember, the heat pump eats $13.89/GJ electricity but pumps out 2.4 GJ for every GJ you feed it. So its cost for heating is only $5.80 or so per GJ of heat delivered to your house. Cheaper than what Energir is paying for fossil gas! (But of course, we homeowners pay a lot more than 5 cents retail per kWh of electricity, so let’s forget about how cheap this could be for a second!)
That $50/GJ wholesale cost for e-methane doesn’t include all sorts of things that will be very, very real costs- we’ve just eliminated them from consideration because we don’t have good estimates for how much they’ll cost, or are too lazy to bother to figure them out. And it doesn’t include profit, or any return on investment for anyone. It assumes the project is run by altruists.
And, as it turns out, it doesn’t matter. Even without those unknown costs in there- all of them at zero- the result is ridiculously expensive e-gas.
So…WTF Would We Ever Do This?
It turns out that the motivation behind this project is likely a mandate by Energir to use a certain percentage of low or zero emissions gas in their network. And they can’t get enough biogas…apparently…even though it is vastly cheaper than this e-gas ever will be.
And the other reason is as clear as day to me: subsidy harvesting. One can imagine not only attracting a substantial “tax credit” from the federal government for making green hydrogen (effectively reducing capital cost by getting the government to pay for it!) , then more still for making green gas out of it- and then it wouldn’t surprise me if they thought they could get CO2 capture credits of some kind here too. Nevermind the fact that any CO2 captured, ends up in the atmosphere when the gas is burned…
Well, there you go, folks. e-methane is an exergy destroying machine on steroids. And if it is built, this TES project will be a very efficient extractor of money out of likely both the federal and provincial governments AND out of the pockets of Energir gas consumers.
What it isn’t, is a sensible decarbonization strategy.
References:
1) Data from real projects for electrolyzer efficiency, proprietary (I can’t share the quotations with you, sorry)
2) Moioli et al, Renewable and Sustainable Energy Reviews, 107, 2019
3) My own calculations for the ultimate conversion efficiency for methanol and methane from CO2 and hydrogen, validated against ref 2) above
4) Experience with e-fuels projects in terms of approach to ultimate energy conversion efficiency, again confidential (most figures in the academic literature are very significantly over-estimated relative to reality)
Disclaimer: This article was written by a human, and humans make mistakes. If I’ve made one, please feel free to call me out on it and to provide references if possible to set me right. I’ll be grateful for the correction.
If I’ve just upset you because I’m shitting on your pet idea, feel free to contact Spitfire Research Inc. They’ll be happy to tell you to piss off and write your own article.