Image credit: Google Gemini
If you know my writing even a little, you’ll know where I stand on this issue. It’s as simple as my meme, but my many articles (especially this one) explain why, in detail. Those articles come with analysis and references and the comments of many experts in the field as an informal peer review too.

https://www.linkedin.com/pulse/distilled-thoughts-hydrogen-paul-martin
So…where does green hydrogen fit? The simple answer is that it fits a) where it can be made truly green b) for applications where we need the special properties of hydrogen as a molecule, for things which are durable in a decarbonized future.
Where can we make hydrogen that is truly green? Since it is an economic myth that we can make affordable hydrogen only from electricity that would otherwise be curtailed, and because the obvious highest value use for green electricity is to replace BLACK electricity (electricity made from fossils without carbon capture), it stands to reason that we can only make green hydrogen where and when the local grid is already as green as we can manage to make it in practical terms. Sadly, at present, that means only in places where the potential to make new green electricity greatly exceeds the ability of people within reach of an HVDC cable (i.e. within a few thousand kilometres by land or sea) to make use of that electricity. Everywhere else, that electricity’s highest value use for decarbonization isn’t to make hydrogen- it’s to use it to displace dirty electricity from the grid.
If we do make some green hydrogen that we think we can afford, it similarly isn’t really “green” unless we use that hydrogen for uses that require hydrogen, and which will survive in a decarbonized economy. Those applications are largely things we use dirty, fossil-derived, high CO2e emission hydrogen for today. And that’s substantially all the hydrogen in the world today- 99%+ of it is made to the tune of over 120 million tonnes/yr, not to waste as a fuel, but we make it from fuels, with CO2 and methane and toxic combustion emissions going to the atmosphere as a result. And by my estimation, the making of this fossil-derived hydrogen- and there’s nothing “gray” about it, it’s blacker than black in terms of emissions- has CO2 emissions greater than those of aviation and almost greater than those of both aviation and shipping combined.
Existing Uses of Hydrogen
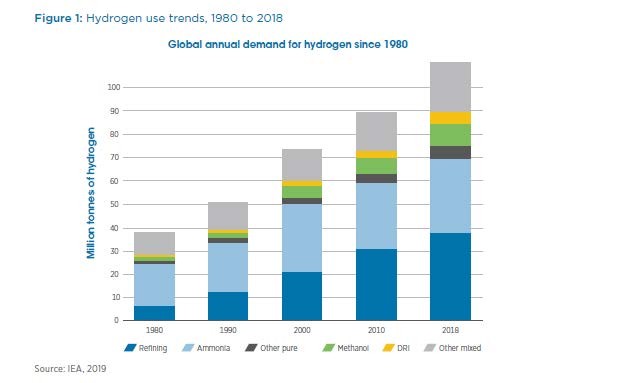
The three major categories of hydrogen demand are ammonia, petroleum refining, and everything else. Each of these consumes about 1/3 of world hydrogen production. The figures for hydrogen production and consumption by sector, both pure and in syngas (mixtures with carbon monoxide or CO) are from the IEA via IRENA and are referenced here:
Ammonia
Ammonia is the toxic gas that is keeping half the humans and their food animals on earth, alive by feeding them. A deeply ironic state of affairs, with huge emissions that we must reduce.
https://www.linkedin.com/pulse/ammonia-pneumonia-paul-martin
We use about 40 million tonnes/yr of hydrogen, substantially all of it blacker than black, to make ammonia, which is the source of all our nitrogen fertilizers and also the base of the entire nitrogen chemicals industry on earth. We make so much ammonia that we humans are fully half the nitrogen cycle on earth. (Note that while we are, with scientific certainty, wrecking the stability of the climate by burning fossils and emitting fossil CO2 to the atmosphere, it is the cumulative effect of doing that over decades which is the problem- despite the fact that we humans and all our activities represent a small fraction of the annual carbon cycle on earth.)
The emissions from ammonia production are not just from the CO2e-emissive process of making the hydrogen that we then react with nitrogen to make ammonia itself, but also from the N2O emissions (a very powerful and persistent GHG) which inevitably result when we apply ammonia, and nitrates and urea made from ammonia, to agricultural soils. We need to improve agricultural practices to reduce those N2O emissions. But we also can and must reduce how much CO2e is generated to make ammonia in the 1st place. And we can do that if we’re smart, by using truly green hydrogen in place of black hydrogen.
So- why aren’t we doing that? Why do we hear almost nothing about projects to make green ammonia to replace black ammonia for use in fertilizers? Yes, there are a handful of such projects in the works, but most green ammonia projects (or dreams of future projects, which are much more common than real projects) are centred around bad ideas like burning ammonia in power plants, or as a ship’s fuel, or cracking ammonia with added heat at destination to make it back into H2 and N2 again. Why is that?
One word, one reason: subsidy. Perverse and ill considered subsidies generate perverse incentives, and tempting people to use a poison gas as a ship’s fuel is in my view, pretty much the definition of a perverse outcome.
No farmer will voluntarily pay more for green ammonia when their competitors can produce the same crops to sell in the same the same markets with cheaper black ammonia. And no, a future where green ammonia is cheaper than black ammonia, even with heavy carbon taxes in place but without subsidy involved, is not something that we can expect in the foreseeable future in my view. While there is hope for something close to cost-competitive to come out of green ammonia projects in the very best locations in the world, the delivered cost will still be a multiple of the cost of black ammonia. Carbon taxes and emission bans are key, and are much preferable to subsidy.
Petroleum Refining
Petroleum refining is at once the source and the consumer of about 1/3 of the world’s hydrogen. And both will shrink to about 15-25% of current levels when we stop burning fossils on purpose as fuels.
Hydrogen is used in refineries to remove toxic sulphur and nitrogen species from petroleum cuts that we will later burn- a process known as “hydrotreating”. The hydrogen ends up as H2S, which is subsequently reacted with oxygen and then broken down into sulphur and water. Hydrogen is also used in “hydrocrackers”, which not only hydrotreat a petroleum feedstock, but also break large molecules into smaller ones. When this kind of chain breakage happens, a carbon-carbon double bond is formed in one of the pieces, which is usually undesirable to the final product’s storage stability and usefulness as a fuel. Hydrogen eases the breakage and also adds hydrogen across that C=C double bond, eliminating the stability problem.
Hydrogen is made in refining or petrochemicals operations such as “plat-forming”, a process by which straight chain hydrocarbons are cyclized and dehydrogenated to make aromatic rings like benzene, toluene etc. Cracking of lighter molecules such as naphtha or ethane and propane to make olefins (compounds like ethylene, propylene and butadiene with C=C double bonds, useful for making polymers) also produces hydrogen as a byproduct.
Yes, in a decarbonized future we will still refine petroleum- just 15-25% of what we currently do. And no, there’s no need to make gasoline and diesel or anything else to burn, in that process. Whether or not we’ll choose to afford to do what’s required, is another story.
https://www.linkedin.com/pulse/refinery-future-thought-experiment-paul-martin-4pfoc
In summary, petroleum refining will greatly shrink in hydrogen demand, and may end up being nearly self-sustaining in hydrogen in the end (or might consume a small amount of green hydrogen). Replacing black hydrogen used in petroleum refining today is therefore not a high priority use for any green hydrogen we think we can afford. We should instead keep the focus on eliminating the burning of petroleum and gas products as fuels.
Everything Else
The other 1/3 of current hydrogen uses is divided among many smaller use cases
Methanol
Methanol today is nearly exclusively made from synthesis gas (mixtures of CO and H2) made from natural gas or from gasifying coal. In a decarbonized future, any methanol we think we may need to burn- as a decarbonized shipping fuel for instance- we can make either using biomass or using biogenic CO2, the former being much cheaper than the latter. Both will need green hydrogen- the former because biomass is deficient in hydrogen, the latter because CO2 basically needs to have one of the two oxygens on it “burned off” by reacting it with H2 to produce water. Green methanol production is a good use of green hydrogen- but we should not reach right away for e-methanol (ie. ultimately made from water, CO2 and electricity) as it will likely be considerably more expensive than bio-methanol with added green hydrogen.
Methanol is also currently used to make many other important chemicals and materials, which are rarely burned- such as formaldehyde, formic acid, acetic acid, certain important polymers, and many others. Methanol is also used as a solvent and antifreeze agent.
Direct Reduction of Iron (DRI)
A small percentage of current world iron production is made from iron ore not in a coal coke-fired blast furnace or basic oxygen furnace, but in a process by which syngas (made from natural gas) is reacted directly with iron ore to form iron metal, CO2 and water. The process, known as direct reduction of iron (DRI) has been demonstrated to be possible using only a very small amount of CO, with almost all of the CO replaced with hydrogen.
It is extremely important to note that iron in DRI is used as a chemical reducing agent (a source of electrons), not as a fuel. It is also important to note that “steelmaking” is already largely an electric process in the developed world. For instance, 70% of steel in the USA is made using electric arc furnaces that are fed largely scrap steel plus some fresh iron. That fresh iron could, in part or maybe even in total, be made by pure hydrogen DRI.
Since the reaction between iron oxides and hydrogen is slightly endothermic, electric heating is also required (unlike when syngas is used). The process of DRI using pure hydrogen has been demonstrated at scale by Hybrit and others, and represents the highest technology readiness level (TRL) way we currently have to nearly eliminate CO2 emissions from iron production. Other options are under development, including molten iron oxide electrolysis and some aqueous electrochemical methods, but all are at a much lower TRL and are at least a decade from at-scale market relevance- if they ever make it to market.
DRI with pure hydrogen is a high value use of green hydrogen, and a potential growth area for hydrogen consumption in a decarbonized future – but only if we do it in locations where both ore and the potential to build new high capacity factor wind plus solar hybrid power sources (or, rarely, excess hydroelectricity) exist close to one another, and nobody nearby needs the electricity that could be sold instead. Western Australia is a perfect example, but there may be many other such locations- perhaps including Labrador in Canada, and Sweden where H2 Green Steel is building a plant to do just this (using hydroelectricity).
I also see substitutions of iron for aluminum, magnesium, wood and other materials which are easier to decarbonize (i.e. at a lower cost per unit strength) than iron may be.
Other Hydrogen Uses as a Chemical
Hydrogen is used in many other chemical reactions, and as a reagent to prevent others from happening. It is used to protect metals from oxidation inside furnaces for operations like sintering or bright annealing. It is used to hydrogenate vegetable and animal oils. It is used as a coolant in turbines and certain electrical equipment. And it is used in a host of reactions such as making halo-acids (HF, HCl, HBr), hydroformylations and many others.
What We Should NOT use Green Hydrogen For
Hydrogen’s current use as a fuel is limited to a very small number of specialty uses such as the upper stages of rockets, vitreous quartz “glassblowing” etc. Notional future uses such as fuelcell vehicle fueling or industrial heating, amount to a trivial fraction of current world hydrogen consumption.
Virtually all the hydrogen currently burned is burned for its energy content and as a waste disposal practice, because it is hydrogen mixed with other gases that are simply not worth the bother and expense of separating from one another. Despite the recent “hydrogen economy” hyperbole, hydrogen is in fact one of the cheapest commodity chemicals on earth, relatively easily made from methane- a molecule which itself has basically no uses other than for the production of heat energy or syngas. Hydrogen is really not worth that much- certainly not enough to make it worth sifting hydrogen from a complex gas mixture when there’s a nearby furnace hungry for fuel. That may change in the future, but it’s unlikely to change much.
Green hydrogen’s role in heating, transport and energy storage is usually a response to the wrong question. When you ask, “what else can I burn, aside from fossils”, hydrogen is a frequently offered, simpleminded answer.

But when you ask the better question: “We can’t burn fossils any more- how can we meet our needs for energy services, without generating fossil GHG emissions?”, the answer is almost never hydrogen.
Making hydrogen from electricity is a step backward in exergy terms- and no, that’s not a typo. That step backwards has a huge cost associated with it. One that, I’m sorry, for energy applications, you are very unlikely to ever fix. You may cope with it out of desperation, breaking your wallet in the process, but that is not at all the same thing.
https://www.linkedin.com/pulse/breakthrough-electrolyzer-efficiency-paul-martin
Green hydrogen’s intrinsic exergy destruction is a feature of its foolish use as a fuel, not a bug. It’s a systematic inefficiency that acts as a multiplier of all the upstream emissions associated with every unit of useful energy services you generate from hydrogen as a fuel- moving a car by a kilometre, or heating your home, or storing a unit of electrical energy, or what have you. There are usually better ways- and usually, those ways use electricity more directly, without the exergy destruction resulting from needlessly involving a molecular middleman and its covalent chemical bond formation and breakage.
https://www.linkedin.com/pulse/how-green-hydrogen-lifecycle-basis-paul-martin-pbhyc
But we’re not done yet- hydrogen has the twin problem of being energy and especially exergy inefficient, but also being ineffective as a fuel. It is difficult to move and store. That kills hydrogen as a practical replacement for some applications which are also not suited to electrification, such as transoceanic shipping and aviation. Those applications will need liquid fuels, and those liquid fuels will be much cheaper to provide as biofuels. Green hydrogen’s role there will be limited to yield improvement, as we’ve already discussed in relation to methanol.
Finally, those few applications for high temperature industrial heating that truly need fire rather than just heat, are rarely a good fit for hydrogen due to its lack of an emissive flame. Biofuels are a better solution for those applications too.
Evidence
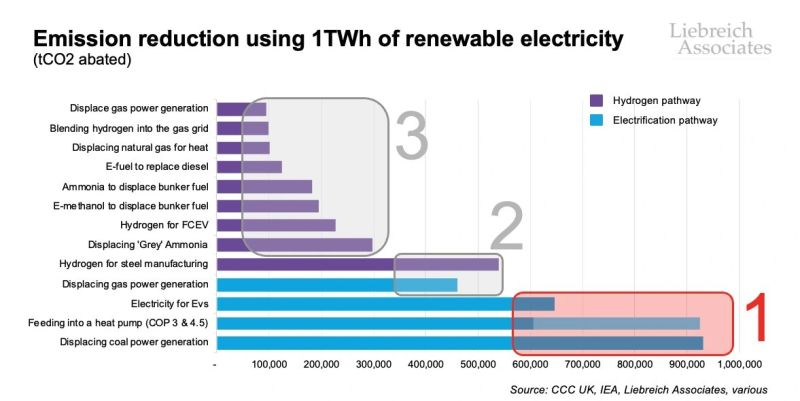
Michael Liebreich has provided a very useful graphic which shows that the highest value uses of green electricity are via the “electrification pathway” in every case where hydrogen is considered as an alternative. And where electricity needs to be made greener, it is far more sensible and economic to do THAT, than it is to try to use that same electricity to produce green hydrogen- even to replace black hydrogen in uses durable post decarbonization. Hence, the famous Drake meme well and properly deserved modification. This point should go without saying, but needs to be said- loudly and often.
Conclusions
It really is as simple as the meme. Green hydrogen should be reserved for applications requiring its special properties as a molecule, where its resulting emissions are absolutely without regret. It should not be wasted as a fuel. And anybody selling you the idea of using hydrogen as a fuel, is selling you a bill of goods- sometimes out of ignorance or poor analysis, but often as a result, at least in part, of the fact that they have an invoice waiting for you in their back pocket- and some of that invoice’s proceeds are headed to their own bank account. That’s not always true, to be clear- there are some true believers in hydrogen as a fuel who literally have no money riding on it. But it was true frequently enough that myself and a number of co-founders saw fit to build the Hydrogen Science Coalition, to provide insights about hydrogen’s true role in decarbonization, from a position of scientific and engineering knowledge, free of any significant financial interest one way or another.
Disclaimer: this article was written by a human, and humans are known to make mistakes from time to time. Let me know, with good references, where I’ve gone wrong, and I will correct my work with gratitude.
If however what you don’t like is that I’ve taken a dump on your pet idea, then please feel free to contact my employer Spitfire Research Inc., who will be most happy to tell you to piss off and write your own article.