There is a popular myth in the marketplace of ideas at the moment: the notion that hydrogen will become a way to export renewable electricity in a decarbonized future, from places with an excess of renewable electricity, to places with a shortage of supply and a large energy demand. It seems that the hydrogen #hopium purveyors are rarely satisfied with the notion that any particular place- my home and native land of Canada for instance- might make enough green hydrogen to satisfy its own needs for hydrogen, but rather, push on to sell the idea that we will become a hydrogen exporter too!
And like all myths, the notion of hydrogen as an export commodity for energy is separated from an outright lie by a couple grains of truth.
The Lands of Renewable Riches
There are places in the world which have huge potential to generate high capacity factor renewable electricity, and which have no significant local use for electricity (hint- that’s not Canada, folks! Any hydroelectricity we have in excess, has a ready market in the USA) This is particularly true of special locations- deserts with oceans to the west- which are also so distant from electricity markets that the option of transporting electricity via high voltage DC (HVDC) is costly and challenging to imagine. Places like Chile, Western Australia, Namibia and other points on the west coast of Africa, come to mind. Remember that high capacity factor renewables are essential if green hydrogen production is ever to become affordable – electrolyzers and their balance of plant are unlikely to get cheap enough to ever make cheap hydrogen from just the fraction of renewable electricity that would otherwise be curtailed.
The Energy Beggars
There are also places in the world with large, energy-hungry populations, on small landmasses, who aren’t particularly fond of their nearest land neighbours: South Korea and Japan come immediately to mind- the option of importing HVDC electricity via a cable which can be “stepped on” by an unfriendly neighbour every time they’re irritated with you is clearly not appealing, if the lessons of the Ukraine war and Russian gas supply are of any use! And there are numerous other places in the world which don’t want the cost and inconvenience of building out huge renewable and storage infrastructure, for renewables with poor capacity factor and hence need broader grids and storage and overbuilding.
These places also have a long history of importing fossil fuels by ship or by pipeline from distant countries- and, usually, a long history of trying unsuccessfully to get un-stuck from that situation for strategic reasons.
The simpleminded approach to decarbonizing their economies is to import chemical energy, just in another form, this time without the fossil carbon- assuming that is both technically possible and affordable- as long as it’s by ship, so they can switch suppliers in an emergency.
Hydrogen Exports to the Rescue!
Matching that obvious source of supply with that obviously thirsty demand, seems a no-brainer. And at first glance, hydrogen seems to fit the bill as a way to connect the two. It is already produced at scale in the world: we make 120 million tonnes of the stuff per year as pure H2 and as syngas, albeit almost all of which is produced from fossil fuels, without carbon capture, right next to where it is consumed.
We do know how to move and store it, though we don’t do much of either. Only about 8% of world H2 production is moved any distance at all, and most hydrogen is consumed immediately without meaningful intermediate storage. And whereas there are about 3,000 miles of hydrogen pipeline in the USA, which sounds like a lot, that compares with 3,000,000 miles of natural gas pipeline in the USA. Most hydrogen pipelines are used for outage prevention among refineries and chemical plants, and to serve smaller chemical users, in “chemical valley” type settings such as the US gulf coast, where you can’t throw a stone without hitting a distillation column. The long distance transmission of hydrogen is, with very few exceptions, basically just not done. It’s not impossible- we do know how to design and build hydrogen pipelines and compressor stations- it just doesn’t make sense to do it, relative to moving something else (natural gas, for instance), and then making the low density, bulky hydrogen product where and when it’s needed.
If you have energy already in the form of a chemical- particularly a liquid- moving that liquid by pipeline is the way to move it long distances with the lowest energy loss, lowest hazard and lowest cost per unit energy delivered. When your energy is already in the form of a gas, it’s almost, but not quite, as good. So at first glance, pipelines look appealing as a way to move hydrogen around- assuming that you already have hydrogen, that is!
The re-use of existing natural gas pipelines for transporting hydrogen, either as mixtures with natural gas or as the pure gas, has been dealt with in another of my papers:
https://www.linkedin.com/pulse/hydrogen-replace-natural-gas-numbers-paul-martin/
…and so we won’t re-hash the argument here. But I concluded, with good evidence:
- The re-use of natural gas long distance transmission pipelines for hydrogen beyond a limit of about 20% by volume H2, is not feasible in most pipelines due to incompatible metallurgy.
- 20% H2 in natural gas represents about 7% of the energy in the gas mixture, and hence isn’t as significant as it sounds in energy or decarbonization terms.
- Hydrogen, having a lower energy density per unit volume than natural gas, consumes about 3x as much energy in transmission as natural gas does in a pipeline, and would require that all the compressors in the pipeline be replaced with compressors of 3x the suction capacity and 3x the power.
We are therefore really talking about using new long distance transmission infrastructure to move hydrogen around. We won’t be able to simply repurpose the old natural gas transmission network, as desperately as the fossil fuel want us to believe we can. We can’t, even if we were to manage to take care of all the problems with the distribution network and all the end-use devices for natural gas that are also not compatible with pure hydrogen.
I had a careful look at a recent academic paper, which compared the shipment of hydrogen and other fuels by pipeline, against the shipment of similar energy via high voltage DC (HVDC):
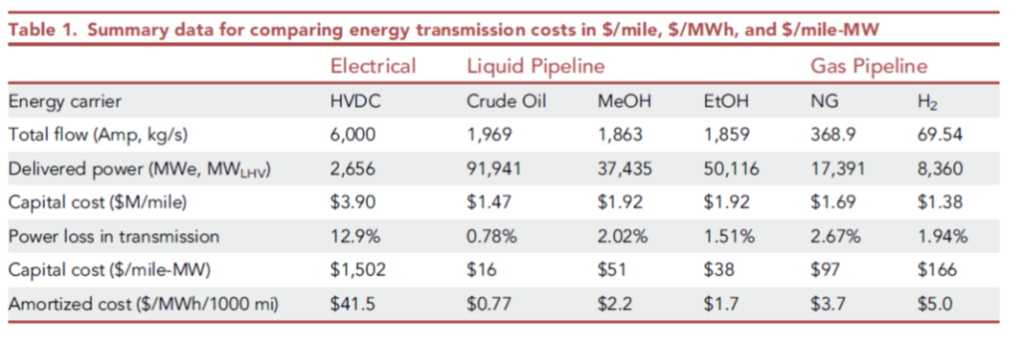
Costs of transmission only, from De Santis, Lyubovsky et al Cell Press 2021 https://www.cell.com/iscience/fulltext/S2589-0042(21)01466-8
However, the paper commits what I have been calling the 2nd Sin of Thermodynamics: it confuses electrical energy (which is pure exergy, i.e. can be converted with high efficiency to mechanical energy or thermodynamic work), with chemical energy (i.e. heat, which cannot), just because they are both forms of energy with the same units. They’re not equivalent, any more than American dollars and Jamaican dollars are equivalent simply because they’re both money, measured in units of dollars! There’s an exchange rate missing…Note in the figure below, electricity and fuels are compared per unit of LHV (lower heating value). Convert that back to equivalent units of exergy and you’ll see that hydrogen, at $2-4/kg, is vastly more expensive as a commodity than the on-shore wind electricity it would presumably be made from to compare with.
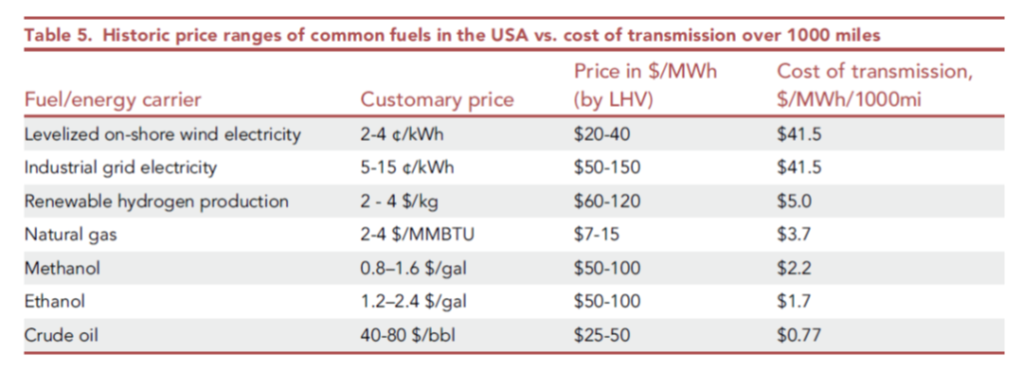
The paper’s authors make other confusing choices, such as running the hydrogen at a considerably lower velocity in the line than indicated by normal pipeline design methods, and these choices affect the conclusions considerably. So whereas the energy loss for H2 versus natural gas should be three times as high per unit of energy delivered, they conclude it is actually lower than for natural gas. The losses stated for HVDC, of 12.9% per 1000 miles, are also considerably over-stated relative to the industry’s metrics of performance (see JRC97720 as just one example).
When you consider that the energy loss involved in just making hydrogen from electricity is on the order of 30% best case (relative to H2’s LHV of 33.3 kWh/kg), and that this energy needs to be fed as electricity (work), it soon becomes quite clear that the cost of transmission by pipeline versus HVDC is quite foolish if what you’re really looking at is the cost to move exergy (the potential to do work) from one place to another. If you start with electricity, the cost of using hydrogen as a transmission medium for that electricity includes an electrolyzer and a turbine or fuelcell at the discharge end of the pipeline. The pipeline itself isn’t actually the controlling variable!
Another paper I recently reviewed; d’Amore-Domenech et al, Applied Energy, Feb. 2021
This paper looked at both subsea pipelines for carrying 2 GW of energy to distant locations, and at 0.6 GW delivery from offshore to onshore locations. This is getting closer to the sort of thing which might be considered to move hydrogen from North Africa to Europe, or perhaps one day from Australia to anywhere else.
It turns out that both subsea pipelines and HVDC cables on the order of 1000 km, already exist. In fact, much longer HVDC lines are currently under study, including one proposed from Darwin, northern Australia, to Singapore, and another from Morocco to the UK.
The paper’s authors assume that HDPE pipe would be used to transmit the hydrogen at electrolyzer discharge pressures of ~ 50 bar(g), to avoid subsea compressor stations ($$$$$). The pipeline loses hydrogen by permeation through the HDPE pipe (resulting in losses of high GWP potential hydrogen to the ocean and hence the atmosphere), and the pipe is increased in diameter along its length as the hydrogen expands due to frictional pressure loss.
Sadly, the paper’s authors also commit the 2nd Sin of Thermodynamics, comparing a MWh of delivered electricity (pure exergy) as if it were worth the same as a MWh of hydrogen higher heating value (HHV). This is a rather glaring error that seems to have passed right through peer review without comment, and it affects the conclusions significantly.
The authors include an 80% (state of the art best case) efficiency for converting electricity to hydrogen HHV at 50 bar(g), and look at this over a 30 yr lifetime.
The energy lost over 30 yrs for HVDC is 1.2×10^4 TJ
The energy lost over 30 yrs for the H2 electrolyzer and pipeline is 1.2x 10^5 TJ, i.e. ten times higher.
Despite this, they conclude that the lifecycle cost of transmitting energy in the form of hydrogen is a little lower for a pipeline than for HVDC at > 1000 km in length. That is, of course, entirely cancelled out by the 50% conversion factor and the cost of the device at the end of the pipe, required to convert hydrogen HHV back to electricity again, which were ignored in the paper entirely. In other words, entirely opposite to their conclusion, their paper leads us to conclude that HVDC is actually considerably cheaper on a lifecycle basis.
For distances longer than 1000 km, the paper concludes that liquid H2 transport is the better option. We’ll deal with that one next…
We won’t even discuss the shipment of compressed gas in cylinders. A US DOT regulated tube trailer carrying hydrogen at 180 bar(g) (2600 psig), i.e. the biggest tank of hydrogen gas permissible currently to ship over US roads, contains a whopping 380 kg of H2. While one day US DOT may permit pressure to increase to 250 or even 500 bar(g), it should be clear that shipping BILLIONS of kilograms of hydrogen as a compressed gas in cylinders across transoceanic distances is just utterly a non-starter.

Liquid Hydrogen (LH2)
Michael Barnard’s article on the subject is well worth a read,
Here’s my stab at evaluating the export of hydrogen as a cryogenic liquid.
Hydrogen becomes a liquid at atmospheric pressure at a temperature of around -249 C, or 24 kelvin, i.e. 24 degrees above absolute zero. At that mind-bogglingly low temperature, it is still not very dense. Whereas compressed hydrogen at 10,000 psig (700 barg) is about 41 kg/m3, liquid hydrogen is only 71 kg/m3. The improvement in energy density per unit volume is not spectacular. And whereas to compress hydrogen from the 30-70 bar pressure at the output of an electrolyzer, to 700 bar(g), can be accomplished for about 10% of the energy in the hydrogen (in the form of work, i.e. electricity, mind you!), liquefying hydrogen takes a mind-boggling 25-35% of the LHV energy in the product hydrogen- again, in the form of electricity to run the compressors- that compares to ~ 10% for liquid methane (LNG).
Take the exergy of the hydrogen itself into account by applying a conversion efficiency of 50% to the hydrogen at destination to convert it back to electricity, and even without the energy involved in transport of the liquid hydrogen (i.e. whatever energy it takes to move the ship etc.), you get a loss on the order of 50-60%, i.e. you are making very poor use of electricity at the source from which you’re making hydrogen and then liquefying it.
Today, we use liquid H2 as a hydrogen transport medium only very rarely. The major uses for liquid hydrogen are cooling NMR magnets, and the upper stages of rockets. That’s about it- there’s no other meaningful use which justifies the extreme complexity and cost of involving a 24 kelvin liquid gas.
The problems of hydrogen liquefaction are considerable, and very technical. First, hydrogen heats up when you expand it, any time you start at a temperature above about -73 C (200 K)- this behavior arises from hydrogen’s unusual negative Joule-Thomson coefficient above 200 K. That means, if you want to liquefy hydrogen, you first have to cool it down considerably as a gas. Generally liquid nitrogen precooling is used for this purpose, necessitating an air liquefaction plant as part of the works. After precooling, the hydrogen can be liquefied by either a helium refrigeration cycle or a hydrogen Claude cycle (where hydrogen itself is the refrigeration fluid).

(image source: Linde)
The energy input required is considerable as a result of the difficulty of rejecting heat to the ambient world when starting at such a low temperature. And although that would be bad enough, hydrogen has another wrinkle: spin isomerization. The electron spins of the two hydrogen atoms in a hydrogen molecule can be either aligned (ortho) or opposite (para). When you condense gaseous hydrogen, you get a mixture of about 75% ortho and 25% para-hydrogen. As the liquid sits in storage, ortho gradually converts to para, releasing heat. And that released heat escapes the only way it can- by boiling hydrogen you’ve spent so much energy to cool and condense. A catalyst is required to carry out the conversion more quickly so the heat can be recovered prior to storage, rather than causing excessive boil-off while the H2 is being stored.
Keeping heat out of liquid hydrogen at 24 kelvin, however, is easier said than done. Vacuum insulated “dewar” type tanks can be constructed, and for applications like this, spherical containers are the optimal shape with the lowest surface area per unit volume. A land-based LH2 dewar tank about as big as you can make it, reportedly has excellent performance, where only 0.2% of the hydrogen in the tank,boils off each day. Any tank smaller than that, or of a less optimal cylindrical shape, allows even MORE than 0.2% hydrogen to boil off per day. And in transit, on a ship or truck, recapture and re-condensation of the boil-off gas is not possible. The best you can do is to burn it, hopefully as a fuel, or if in port, to just burn it to prevent it from becoming a greenhouse gas- H2’s global warming potential (GWP) is at least 11x as great as CO2 on the 100 yr time horizon and it is even higher on the relevant 20 yr time horizon.
Once you get to the size of tank possible to put on a truck, 1% boil-off per day is about the best you can do. Want to make it worse? Just use a smaller tank!
Hydrogen’s low density, even as a liquid, is another problem. Liquid hydrogen, at 2800 kWh/m3 HHV, contains only about 44% of the HHV energy per unit volume of liquid methane (6300 kWh/m3), i.e. LNG. On an LHV basis, i.e. if we need work or electricity at the destination instead of heat, it’s even worse- 2364 kWh/m3 for hydrogen versus 9132 kWh/kg for LNG, i.e. about ¼ the energy density per unit volume. That means either larger energy cargo ships, or several ships to carry the same amount of energy- even if boil-off is managed.
Converting Hydrogen to Other Molecules for Shipment
Confronted with these obvious difficulties, which make hydrogen rather a square wheel for the transport of energy across transoceanic distances, hydrogen proponents don’t give up! Naturally, they try to shave the corners off hydrogen’s square wheel by converting it to another molecule with more favourable transport properties. The four main candidates are ammonia, methanol, liquid organic hydrogen carriers (LOHCs), and metal hydrides. We’ll take these one at a time.
Ammonia
While making green ammonia to replace the black ammonia we rely on to feed about half the humans on earth is inarguably a high merit order use for any green hydrogen we might afford to make in the future, some have gone on to suggest ammonia as a vector by which hydrogen itself may be transported.
Ammonia is discussed in some detail in my paper here:
https://www.linkedin.com/pulse/ammonia-pneumonia-paul-martin/
The advantage is that it is made from nitrogen which can be collected anywhere from the air. The downsides are many:
- Heat is released at the point of manufacture, where energy is already in excess, hence it is likely this energy will be wasted
- The Haber-Bosch process, while efficient after ~ 110 yrs of optimization, must be operated continuously to have any hope of being economic. It is high pressure and high temperature, and hence not suitable to cyclic operation as energy supply rises and falls. This necessitates considerable hydrogen storage if the feed source is renewable electrolysis
- Breaking ammonia part again to make hydrogen takes heat, at the place where you’re short of energy, and at fairly high temperature (so waste heat from fuelcells isn’t likely to be useful)
- Ammonia is a poison to fuelcell catalysts
- When burned in air, ammonia generates copious NOx, requiring yet more ammonia to reduce these toxic and GWP-intensive gases back to nitrogen again (NOx consists of N2O- a 300x CO2 GWP gas which is persistent in the atmosphere, NO- a transient species, and NO2, the toxic one which is water soluble and not persistent in the atmosphere but a precursor of photochemical smog etc. Burn ANYTHING- hydrogen, ammonia, gasoline, your old boss’s photograph etc., and you get all three)
- Ammonia itself is dangerously toxic, especially in aquatic environments
- Large shipments of ammonia would be insidious targets for terrorism
- Cycle efficiencies, starting and ending with electricity, for processes involving ammonia, are on the order of 11-19%, meaning that you get 1 kWh back for every 5-9 kWh you feed
Because substantially all ammonia used in the world is of fossil origin, made from black hydrogen which itself is made from fossils with methane leakage and without carbon capture, and its use literally feeds the people of the earth, I see any use of ammonia as a fuel before black ammonia is replaced with green ammonia, as being basically energetic vandalism. It has an objective clearly different than that of decarbonization in my view.
Methanol
Methanol, which is currently exclusively made from natural gas or coal by gasification to produce syngas (mixtures of H2 and carbon monoxide), can also be made by producing an artificial syngas by running the reforming reactions backward- starting with CO2 and H2 and catalytically producing CO and H2O. While that energy loss, generating water by basically “un-burning” CO2, is substantial, as long as a CO2 source of biological or atmospheric origin can be used, methanol has a series of attractive properties:
- It is a liquid at room temperature, not just a liquefied gas, so its cost of storage is very low per unit energy (though tanks do need inerting, which is unnecessary for gasoline or diesel)
- It is toxic, but nothing even close to the toxicity of ammonia
- Its energy density is lower than that of gasoline and diesel, but once made, it is considerably more favourable as an energy transport or storage medium than ammonia or hydrogen
- It may be reformed at modest conditions back to synthesis gas again
- It is a versatile chemical used to make many other molecules, including durable goods such as plastics, and if we are not foolish enough to burn those materials at end of life, it can be a mechanism for carbon sequestration
The big challenge for methanol is that source of CO2. Direct air capture wastes too much energy in a needless fight against entropy, so forget about it as a source of CO2 to make methanol in my opinion. Unless a concentrated source of non-fossil CO2 (a brewery, anaerobic digester or biomass combustor) is colocated with the source of electricity and hence hydrogen, the shipment of liquid CO2 by sea to make methanol from, replicates many of the economic challenges of LNG and liquid hydrogen.
While making green methanol is also a clearly no-regrets use of any green hydrogen we may happen to make, methanol as an “e-fuel” is a challenging issue for the above-noted reasons. Obtaining decent economics per delivered joule would seem very challenging indeed. Therefore, the hopes of companies like Maersk that they will be able to fuel their ships on fossil-free methanol in the near future, seem perhaps decades premature at best.
The use of methanol as a vector for the transmission of hydrogen for use as hydrogen, makes no sense to me at all. Reforming the resulting CO back to CO2 and more H2 again using water is possible, but too costly and lossy to make energetic sense to me.
Liquid Organic Hydrogen Carriers (LOHCs)
These are liquid organic molecules like methylcyclohexane, which can be dehydrogenated to produce hydrogen and toluene. The toluene, also a gasoline-like liquid, can be shipped back to wherever hydrogen is in excess, and hydrogenated to produce methylcyclohexane again. Numerous molecule pairs are candidates, each with its suite of benefits and disadvantages.
The big disadvantages of LOHCs are similar to those of ammonia:
- Parasitic mass is considerable – for MCH/toluene, only 6% of the mass of MCH is converted into hydrogen at destination, and the other 94% of the mass has to be shipped in both directions. On this basis alone, LOHCs are not good candidates as transportation fuels (i.e. fuels for use to move ships, trucks etc.) in my view
- Like with ammonia, heat is produced at the place where you have energy in excess, and energy is required (again at high temperature) to supply the endothermic heat of dehydrogenation at destination. The temperatures required are too high for waste heat to be used
- There will inevitably be some loss of the molecules in each step. Yields will never be 100%
- Considerable capital and operating/maintenance cost will be required at both ends, for the hydrogenation/dehydrogenation equipment. These are chemical plants, not simple devices like fuelcells or batteries, and hence they will be economical only at very large scale if ever
LOHCs don’t seem to have a good niche in my view. They are useless as sources of hydrogen for transport, below the size of perhaps a ship. While some, such as Roland Berger in a recent report:
https://www.rolandberger.com/en/Insights/Publications/Transporting-the-fuel-of-the-future.html
…tend to conclude that LOHCs are a better way to do “last mile” transport of hydrogen under certain circumstances than some of the other options, that is again really a desperate reaction to the impracticality of hydrogen itself as an energy distribution vector, rather than a vote of confidence in the technology itself.
Solid Metal Hydrides
Hydrogen reacts with both the alkali metals (Li, Na) and alkaline earth metals (Ca, Mg) as well as with aluminum and other elements, to form hydrides, i.e. where hydrogen is in the form of H- ion. These hydrides can form at the surface of the metals, providing a means of “chemi-sorption” for the storing of hydrogen at lower pressures than that required for pure compressed gas. However, the cost of the lower storage pressure is greatly higher (parasitic) mass, i.e. useless for transport applications, and the need to use heat (generally provided by electric heating) to desorb the hydrogen when required.
The hydrides themselves can also be made as pure solid substances, such as “alane” (AlH3), magnesium hydride (MgH2) or NaBH4 (sodium borohydride). These metal hydrides react with water, producing twice as much hydrogen as is found in the original hydride molecule. For instance:
MgH2 + 2 H2O ⇒ 2 H2 + Mg(OH)2
Sadly, there’s the rub: aside from the considerable problem of parasitic mass, in each case, the re-formation of the original hydride involves two steps:
- Production of the metal again from its hydroxide, and
- Production of the hydride by reaction with hydrogen at high temperature and pressure
The energy cycle efficiency of all such schemes involving metal hydride reactions with water are therefore negligible, tending to be in the single digits, because the process of re-making the metal and then the hydride is so energy-intensive. Wasting 10 joules merely to deliver 1 joule at destination is not something we’re going to do at scale, or at least that’s my hope!
Conclusions
The export of hydrogen, either as hydrogen itself or as molecules derived from hydrogen for use as fuels directly or as sources of hydrogen to feed engines or fuelcells, seems to be an idea which although technically possible, is extremely difficult to imagine becoming economic. The energy losses and capital costs and other practical matters standing in the way of hydrogen or hydrogen-derived chemicals being used as vectors for the transoceanic shipment of energy, seems to be rather more a result of #hopium addiction being spread by interested parties, than something derived from a sound techno-economic analysis.
What Should We Do Instead?
It’s clear to me that the opportunity of high capacity factor renewables from hybrid wind/solar installations along the coasts in places like Chile, Western Australia etc. is considerable, and so is the potential for these green energy resources to decarbonize our society.
In my view, however, we’re thinking about it wrong.
We should be thinking about Chile, western Australia etc., becoming hubs for the production of green, energy-intensive molecules and materials- things that we need at scale, which represent large GHG emissions because we currently make them using fossil energy or fossil chemical inputs. The list includes:
- Ammonia, and thence nitrate and urea, for use as fertilisers (NOT as fuels!)
- Methanol, for use as a chemical feedstock, again not as a fuel
- Iron (hydrogen being used to reduce iron ore to iron metal by direct reduction of iron (DRI), which can then be made into steel at electric arc mini-melt mills wherever the steel is needed
- Aluminum, and perhaps one day soon, magnesium too- neither of which involve hydrogen really, but both of which will need electricity in a big way if we want to decarbonize them
- Cementitious/pozzolanic materials- though these are such bulky and low value materials that shipment across transoceanic distances is hard to imagine we’ll be able to afford
- who knows- maybe diamonds and oxygen! (Just kidding!)
For locations such as north Africa, the obvious solution is to skip the hydrogen and indeed the molecular middleman entirely, and simply to export electricity via HVDC directly to Europe. Although that doesn’t address the need for energy storage, the resources predicated for the manufacture of economical green hydrogen already suggest high capacity factor, and proximity to the equator makes their seasonal variation considerably lower as well. Clearly, in my view, making hydrogen simply to permit electricity to be stored for later use is very hard to justify, given the best case cycle efficiency of hydrogen itself- without hydrogen long distance transport and distribution taken into account- is on the order of 37%. That is far too lossy a battery to be worth major investment. Drop that even further by adding lossy things like hydrogen liquefaction or interconversions to yet other molecules and it looks just too bad to take seriously.
What About Fossil Energy Importers?
Countries like Japan and South Korea, frankly, are in big trouble in a decarbonized future, especially if they make themselves dependent on importing energy in the form of hydrogen or hydrogen-derived molecules. What kind of cars they drive is really irrelevant: the energy-intensive industry that is the basis of their economies, will simply need to move offshore, given that their economic competitors would be using energy which costs 1/10th as much per joule, and using that energy directly rather than through a lossy middleman. Either that, or they’ll need to switch to a service economy and focus on extreme energy conservation- which might be best.
However, what concerns me is that neither the Japanese nor the Koreans are ignorant in these matters. If I saw both countries building out renewable offshore wind generation like mad, or even going nuts building new nuclear plants, perhaps I’d believe that their interest in decarbonization via hydrogen was truly in earnest, to sop up even at great cost, the residual that they can’t manage to supply locally as electricity. Rather the focus on hydrogen looks more like an attempt to put off the energy transition until some future date when hydrogen becomes “economic” as an option, burning fossils and fooling around with meaningless pilot projects (JERA burning ammonia in 30% efficient coal-fired power plants, anyone? Or worse still, this brown coal gasification with liquid hydrogen shipment nonsense?) in the meantime. Because, frankly, looking at the various importation options, the future in which hydrogen as an energy transport vector becomes “economic” across transoceanic distances is likely “never”, relative to more sensible options.
Disclaimer: whereas I always try to be accurate, I’m human and therefore fallible. If you find anything wrong in my article, which you can demonstrate to be wrong via good, reliable references, I’ll be happy to correct it. That’s why I publish on a vehicle like LinkedIn, rather than in journals that remain unedited and therefore preserve my errors in amber!
Oh, and if you don’t like my opinion on these matters, by all means feel free to contact my employer, Spitfire Research Inc.
The president (i.e. myself) will be happy to tell you to get lost and write your own article, with even better references, if you disagree.